Microwave Heating - Mechanism and Theory
Microwaves are important and powerful tools found in laboratories across the world, applied beyond reheating leftovers and across varying chemical applications. With the ability to heat efficiently, precisely, and safely, laboratory microwaves benefit chemical-synthesis, material-digestion, and consumer good-testing processes. To understand the origin of these benefits, let’s take a closer look into microwaves and how they generate heat.
What are microwaves?
A microwave is a low energy electromagnetic wave with a wavelength in the range of 0.001 – 0.3 meters and a frequency in the range of 1,000 – 300,000 MHz (Figure 1). Laboratory (and household) microwave instrumentation almost exclusively operate with microwaves at a frequency of 2450 MHz (or 12.2 cm wavelength).
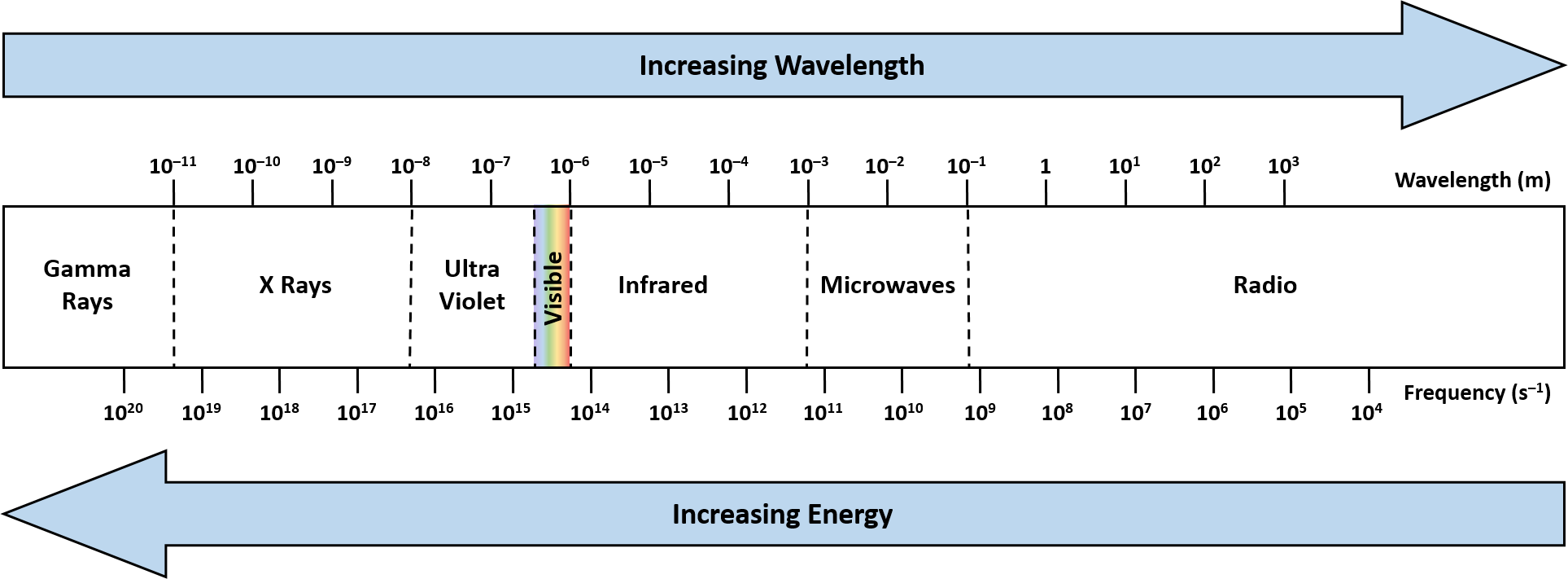
Figure 1. Electromagnetic spectrum
A microwave, like any other electromagnetic wave, travels at the speed of light (300,000 km/sec) and consists of two perpendicular oscillating fields: an electric field and a magnetic field (Figure 2). Microwave photon energy is relatively low (0.03 – 0.00003 kcal/mol), affecting only kinetic molecular excitation.
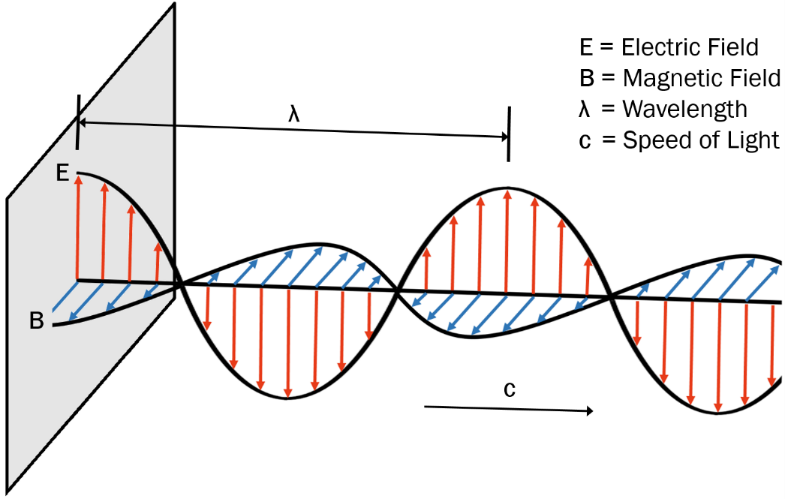
Figure 2. Components of a microwave
How do microwaves generate heat?
In the case of microwaves, the electric field is primarily responsible for generation of heat, interacting with molecules via two modes of action: dipolar rotation and ionic conduction (Figure 3). In dipolar rotation, a molecule rotates back and forth constantly, attempting to align its dipole with the ever-oscillating electric field; the friction between each rotating molecule results in heat generation.
In ionic conduction, a free ion or ionic species moves translationally through space, attempting to align with the changing electric field. Like in dipolar rotation, the friction between these moving species results in heat generation, and the higher the temperature of the reaction mixture, the more efficient the transfer of energy becomes. In both cases, the more polar and/or ionic a species, the more efficient the rate of heat generation.
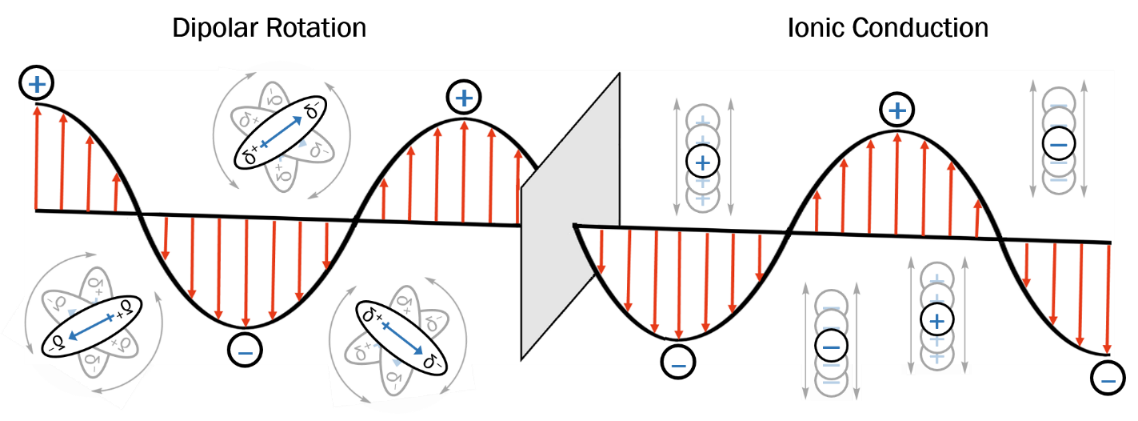
Figure 3. Mechanisms of microwave heating: dipolar rotation and ionic conduction
Because microwaves interact directly with the contents of a reaction mixture, energy transfer occurs more efficiently than with conventional heating techniques (Figure 4). Conventional heating techniques rely on thermal conductivity, where heat is transferred first from source to vessel, and then from vessel to solution. This is a slow and inefficient method of heat transfer, where differing thermal conductivities complicate temperature control abilities and lengthen achievement of thermal equilibrium.
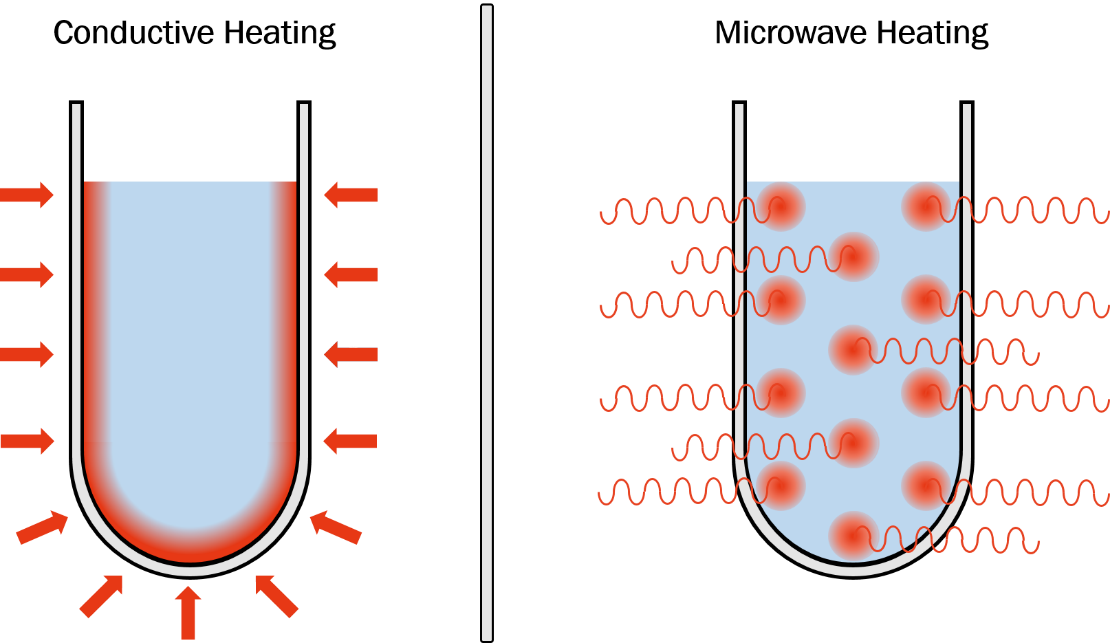
Figure 4. Approaches to heating: conductive heating and microwave heating
Instead of relying on varying thermal conductivities, microwaves instantly heat any solvent, solute, or material in solution through dipolar rotation and/or ionic conduction, resulting in a more efficient, more precise, and safer mode of heating.
References
(1) Galema, S. A. Chem. Soc. Rev. 1997, 26, 233–238.
(2) Brittany, H. (2002). Microwave Synthesis: Chemistry at the Speed of Light. Matthews: CEM Publishing, pp.11-27.
