Heterocyclic Chemistry
Heterocyclic chemistry is another area of importance in synthetic chemistry. A large number of natural products and target drug compounds contain a heterocyclic core. Synthetic routes toward these compounds are usually quite challenging. Microwave-induced heterocyclic chemistry has been extensively examined with pyrimidine derivatives49,125,130,131,169,238-251, pyrroles252-257, pyridines59,64,68,108,258-274, β-lactams11,111,275-286, indoles287-298, γ-carbolines299, quinolines and quinolones47,96,110,112,113,118,300-303, quinazolines67,304-309, imidazoles133,155-156,258,264,265,310-327, other azole/azoline derivatives57,58,64,71,106,107,109,119,133,327-348, furans349-353, and 1,3-dipolar cycloadducts61-63,155,157,177,355-378.
The Biginelli three-component condensation reaction is a one-pot synthesis to dihydropyrimidines. These heterocyclic systems contribute to enhanced pharmacological efficiency in a variety of biological effects, including antiviral, antitumor, antibacterial, and anti-inflammatory activities. With normal conventional heating, these reactions can take approximately 24 hours for complete transformation with only low to moderate yields. Upon microwave irradiation, the Biginelli reaction was successfully completed in five minutes, with 60-90% yields (Scheme 48).354
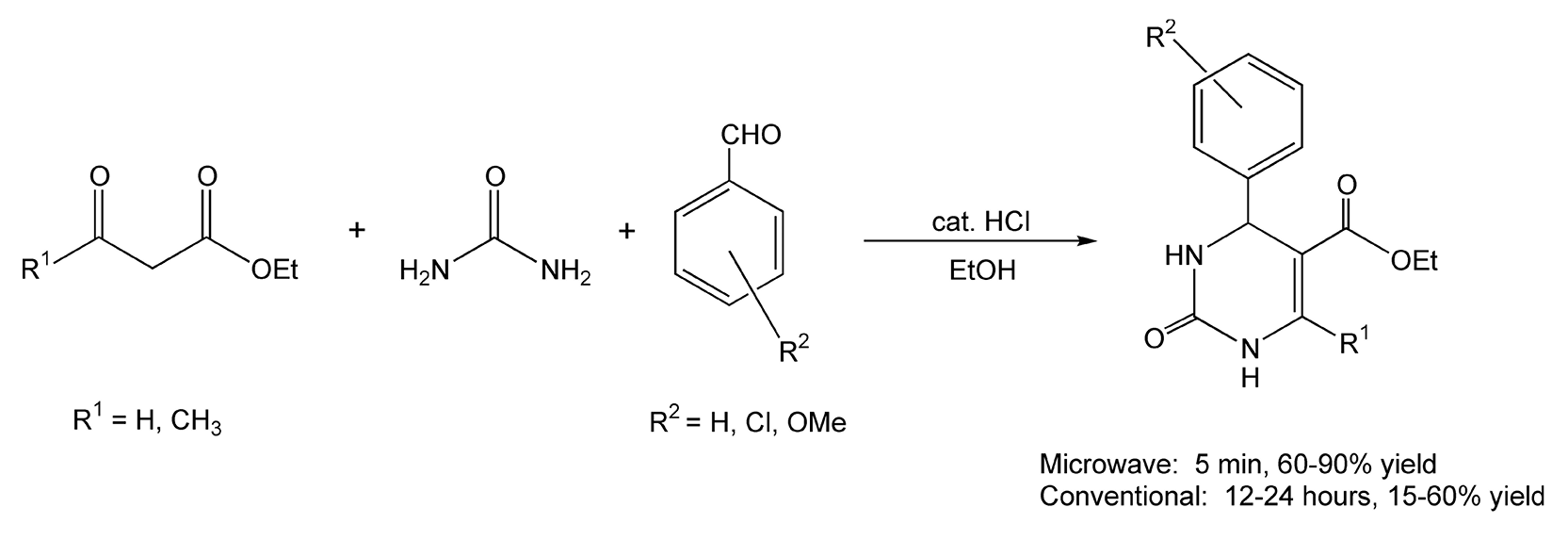
Scheme 48
The Paal-Knorr condensation/cyclization reacts 1,4-diketones with primary amines to form N-substituted pyrroles. This synthesis requires at least twelve hours of prolonged thermal heating and added Lewis acids to activate the diketones. With microwaves, transformation occurred in anywhere from 30 seconds to two minutes with very high yields (75-90%) (Scheme 49).255
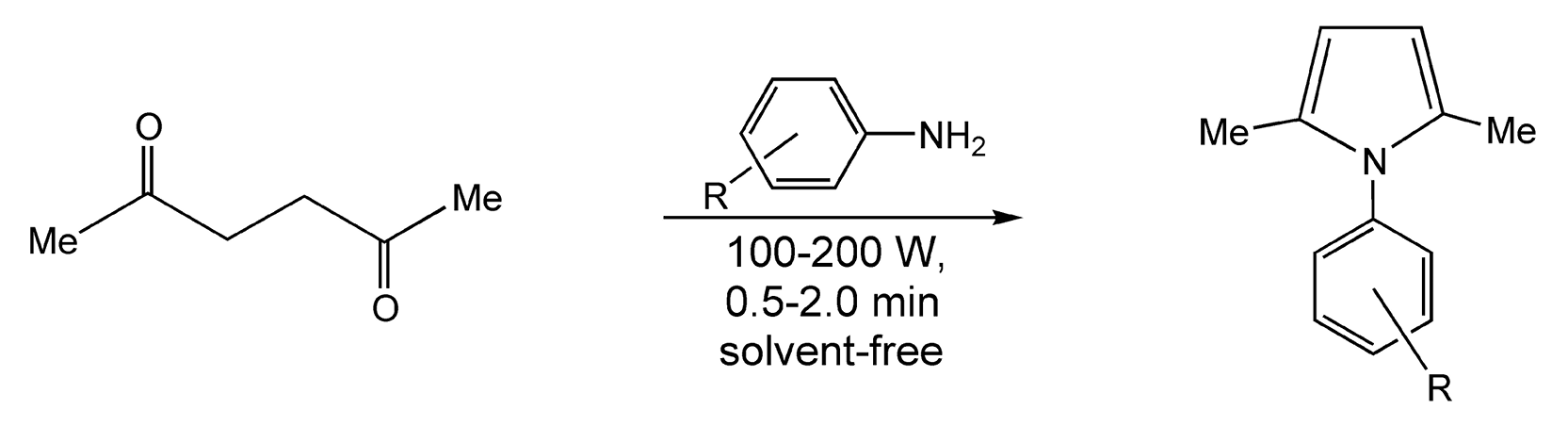
Scheme 49
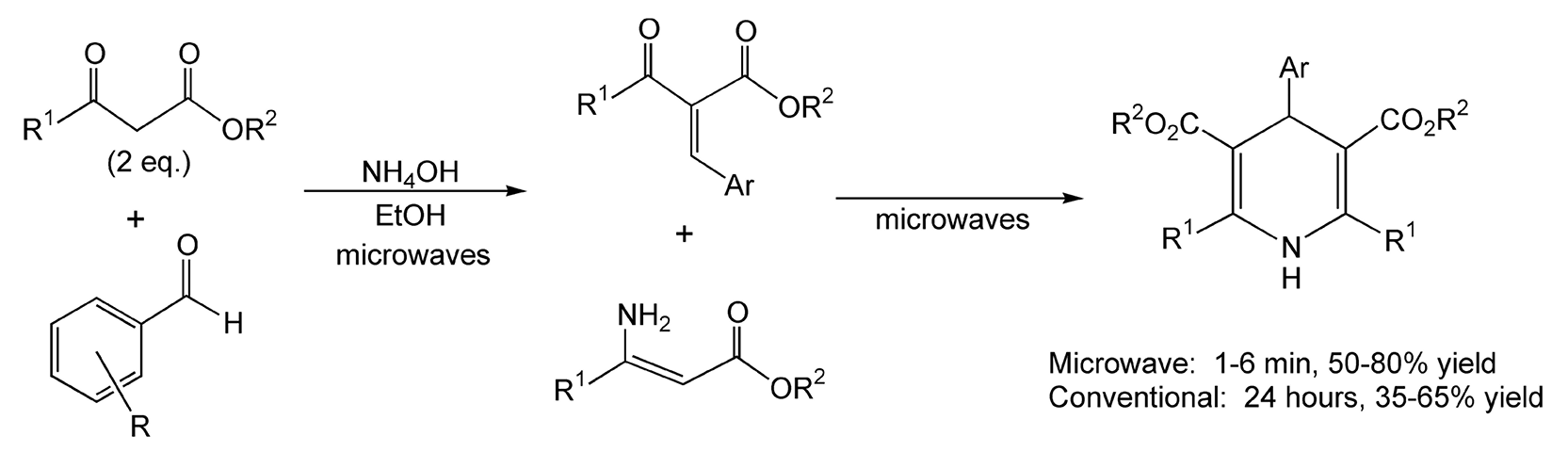
Scheme 50
Substituted dihydropyridines are known to be calcium channel blockers and are quite biologically active. They can be synthesized via the one-pot Hantzsch pyridine reaction. In this particular reaction, an aldehyde, two equivalents of a β-ketoester, and ammonium hydroxide are combined in the same reaction vessel. One equivalent of the β-ketoester and the aldehyde undergo an aldol condensation. The other equivalent reacts with the ammonium hydroxide to yield an enamine. The final transformation, as shown in Scheme 50, results in a dihydropyridine derivative. Classical thermal heating takes over 24 hours, whereas these reactions occur in five minutes or less with microwave irradiation.259,260,267
For decades, synthetic and medicinal chemists have been greatly interested in β-lactams. These four membered chiral heterocycles are versatile synthons in natural product synthesis. Additionally, they can easily undergo rearrangements to yield other heterocyclic and acyclic compounds. Bose and coworkers have done extensive research on microwave-induced β-lactam synthesis.11,278,279,282,283,285 Their early work on β-lactams via conductive heating led to extremely low yields. Utilization of microwave heating for five minutes on an α,β-unsaturated acid chloride and a Schiff base (imine) provides β-lactams in 65-70% yield (Scheme 51). Another synthetic strategy to β-lactams utilizes di-azoketones (Scheme 52).275 Upon irradiation with microwaves, the diazoketone compound is transformed into a ketene, which then rapidly cyclizes with the imine to yield a β-lactam. Classical conditions rarely yielded a product, whereas microwave irradiation produced 60-80% yield of β-lactam.
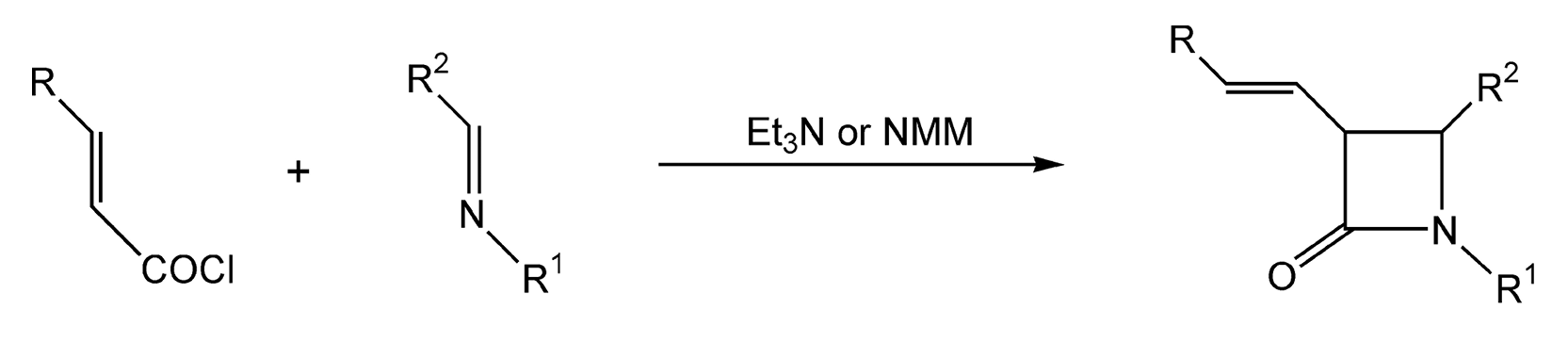
Scheme 51
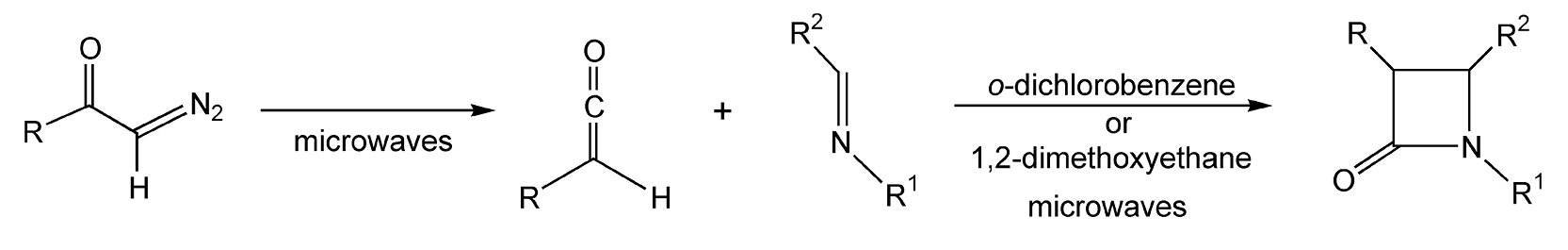
Scheme 52
The Fisher indole cyclization is a one-pot reaction to substituted indolic compounds. This powerful synthetic reaction utilizes an arylhydrazone and an aldehyde or ketone. After a [3,3]-sigmatropic rearrangement, elimination of ammonia yields an indole. Microwave irradiation greatly accelerates (385-fold rate enhancement) this rearrangement, resulting in successful completion of the reaction in less than 30 seconds (Scheme 53).294-296
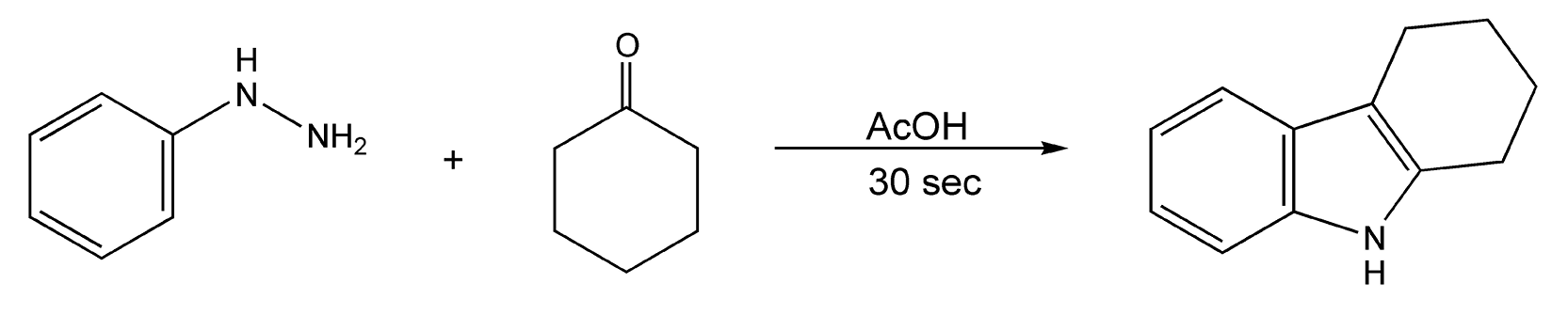
Scheme 53
The Graebe-Ullmann synthesis, which yields γ-carbolines, is another one-pot reaction. This two-step procedure firsts reacts benzotriazole with a 4-chloropyridine followed by polyphosphoric acid addition to produce thermolytic ring closure. Traditionally, both steps require extremely high temperatures for reaction to occur, and the yields are very dependent on temperature control. With microwave heating, the first step occurs in ten minutes (160 W), plus an additional five minutes (or until nitrogen evolution ceases), after the addition of polyphosphoric acid (Scheme 54).299
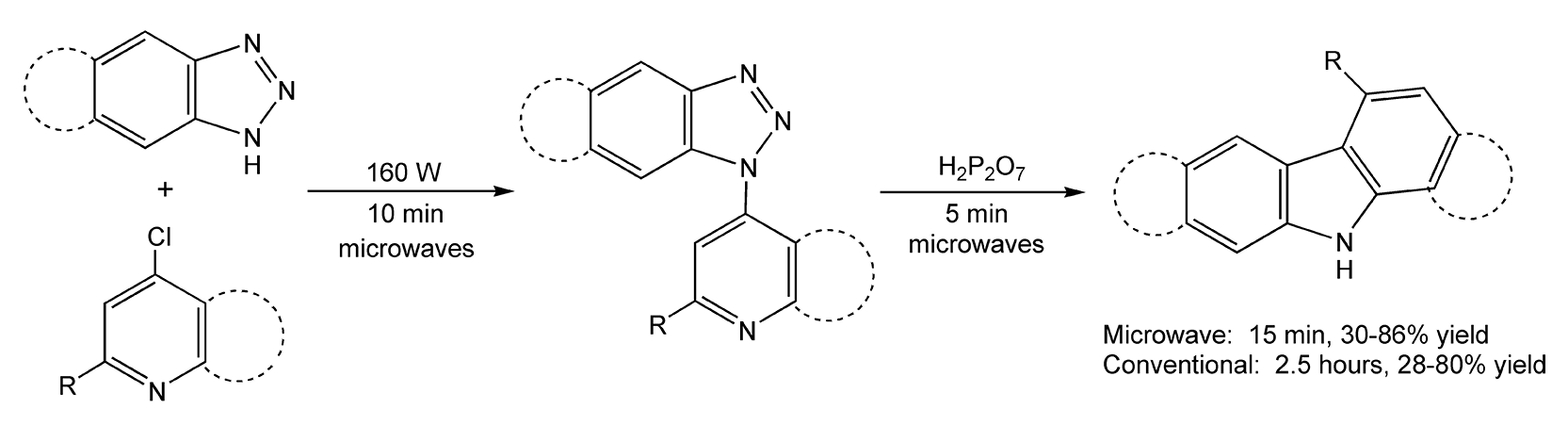
Scheme 54
Quinolines and quinazolines are hetero-bicyclic ring systems containing one and two nitrogen atoms, respectively. These compounds and their derivatives have been shown significant interest by medicinal chemists because of the numerous natural products and potential drug compounds that contain their heterocyclic core. The classic Skraup quinoline synthesis requires large amounts of sulfuric acid and high temperatures, which can be quite a violent combination, and usually does not produce satisfactory yields. With microwave irradiation, it has been reported that an aniline, an alkyl vinyl ketone, and indium(III) chloride (catalyst) on silica gel provide 4-alkylquinolines in 80-90% yield (Scheme 55).300 Quinazolines can be synthesized from an N-arylimino dithiazole derivative and sodium hydride in refluxing ethanol. Reaction times were greatly reduced from 40 hours to two hours with microwave energy (Scheme 56).304
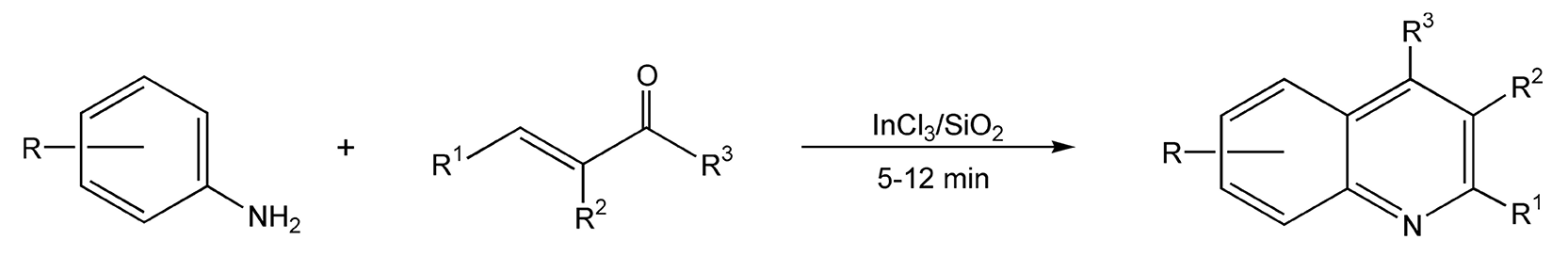
Scheme 55
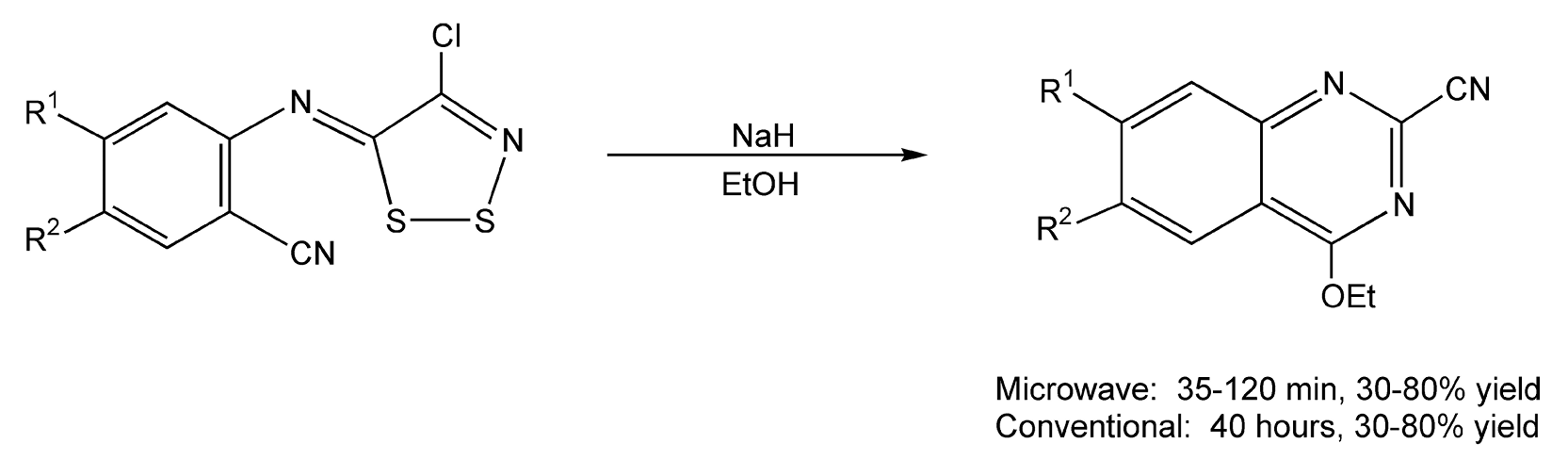
Scheme 56
Azole derivatives — which include imidazoles, oxazoles, thiazoles, and tri/tetraazoles — are five-membered rings containing at least two heteroatoms, one being nitrogen. Once again, these compounds contain very important heterocyclic cores that are common in drugs and natural products. Benzimidazoles, -oxazoles, and -thiazoles can be easily synthesized from o-arylenediamines or other o-arylene heteronucleophiles and a substituted acetylketene diethyl acetal with microwave irradiation (Scheme 57).327 No reaction occurred with thermal heat.
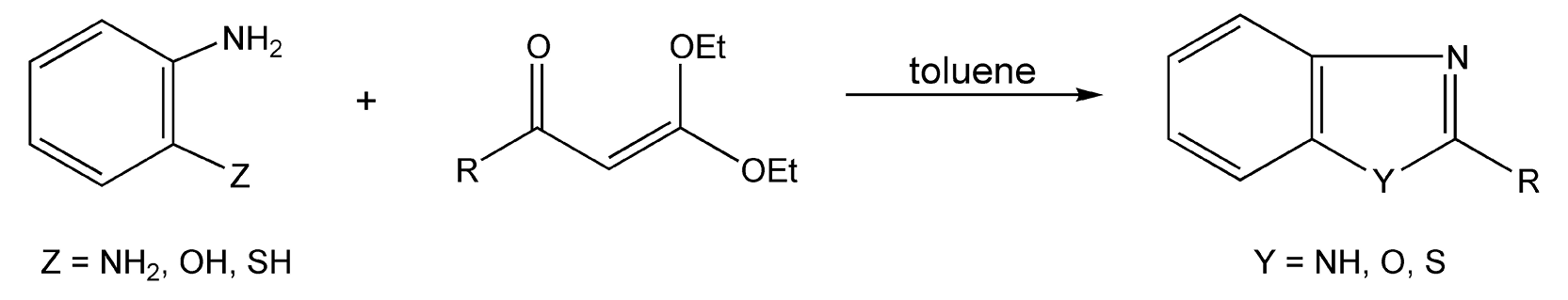
Scheme 57
Another route to substituted imidazoles can be accomplished by condensation of a 1,2-dicarbonyl compound with an aldehyde and ammonia. Classically, this reaction requires four hours of intense heating in acetic acid. With microwave irradiation, imidazoles were produced in 20 minutes (Scheme 58).316
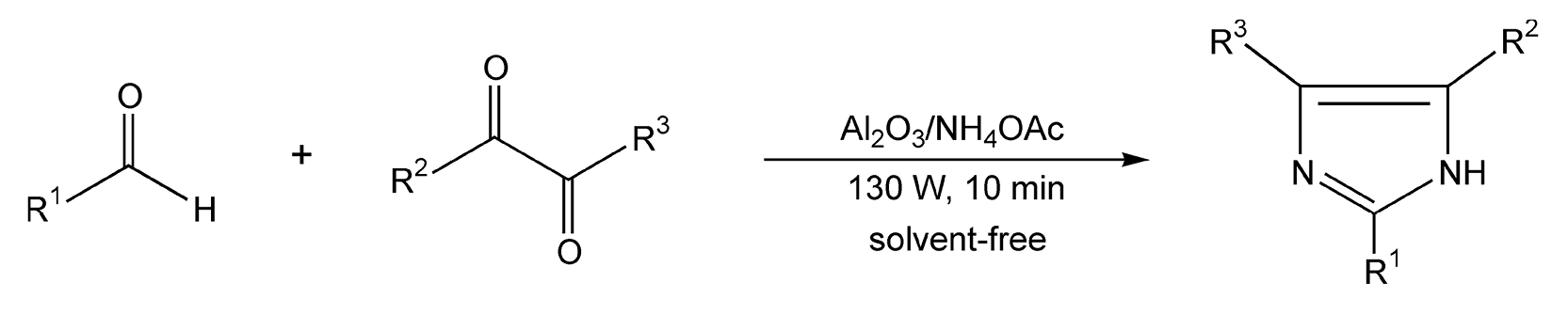
Scheme 58
Triazoles and tetrazoles contain, as their prefixes indicate, three and four nitrogen atoms, respectively. Treatment of arylnitriles with hydrazine dichloride and hydrazine hydrate in ethylene glycol provides substituted 1,2,4-triazoles. Conventional methods require 45-60 minutes of intense heating and provide moderate yields. Microwave irradiation produced the triazole products in about five minutes (Scheme 59).345 Tetrazoles can also be synthesized from nitriles, these via palladium-catalyzed cyanation of organo bromides with zinc cyanide. Subsequent addition of sodium azide and ammonium chloride yield tetrazoles. Conductive heating usually takes from seven hours to four days, while microwaves yielded product in 15 minutes (Scheme 60).348
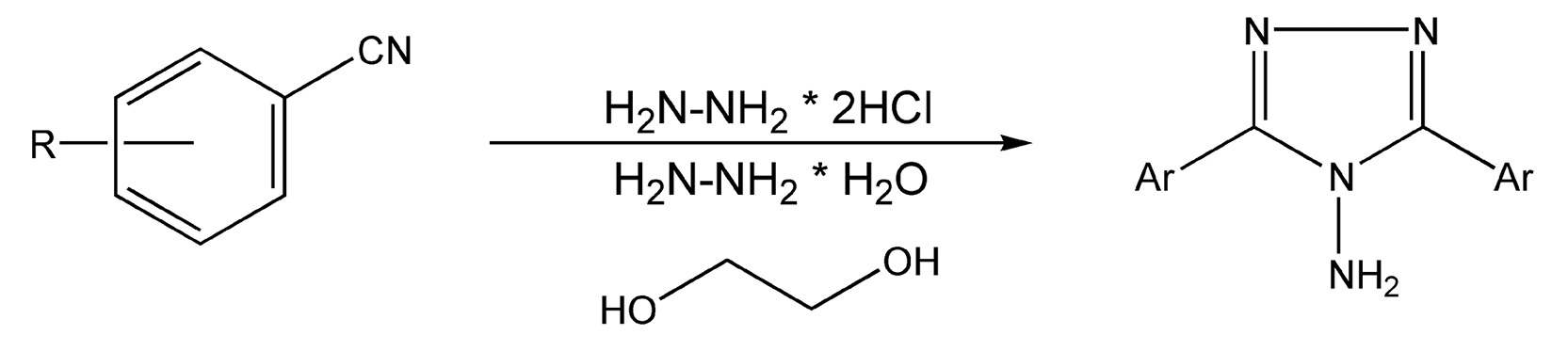
Scheme 59
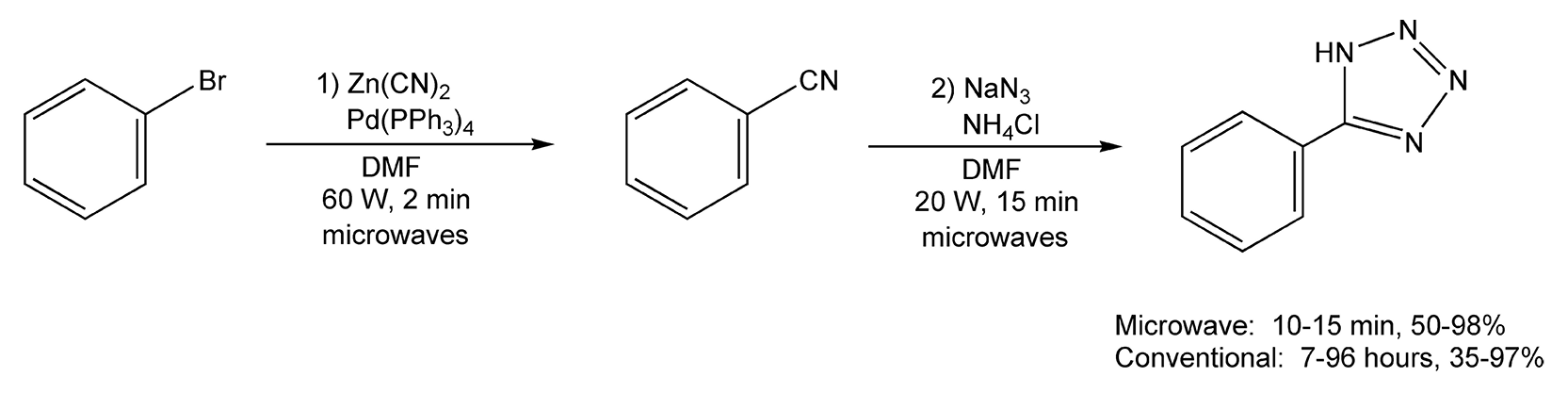
Scheme 60
Furans also contain a common heterocyclic core that is seen in many natural products and drug compounds. Diepoxides, in the presence of sodium iodide, rearrange to form substituted furan ethers. Using thermal heat, this reaction proceeds in five hours with only a 43% yield. With microwave irradiation, rearrangement only took five minutes with an 88% product yield (Scheme 61).352 Additionally, ferrocenyl substituted acrylaldehydes react with a β-hydroxy (or thiol) ester to yield furans (thiophenes) in two minutes under microwave heating (Scheme 62).353 Conventional methods require 24 hours of reflux.

Scheme 61
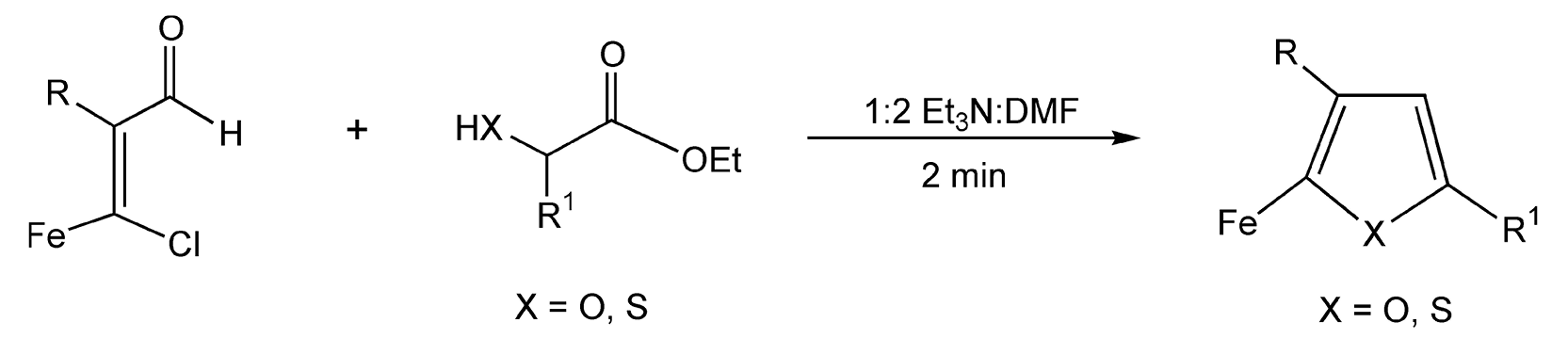
Scheme 62
A 1,3,4-oxadiazole synthesis, as shown in Scheme 63, was executed in two steps.354 The second stage of this reaction was performed, first, with conventional heating methods at 150 °C for 90 minutes. In a separate reaction using microwave energy, the second stage was completed at the same temperature, but it only took five to ten minutes. This reaction was 100-fold faster, even though the measured bulk temperatures were the same.
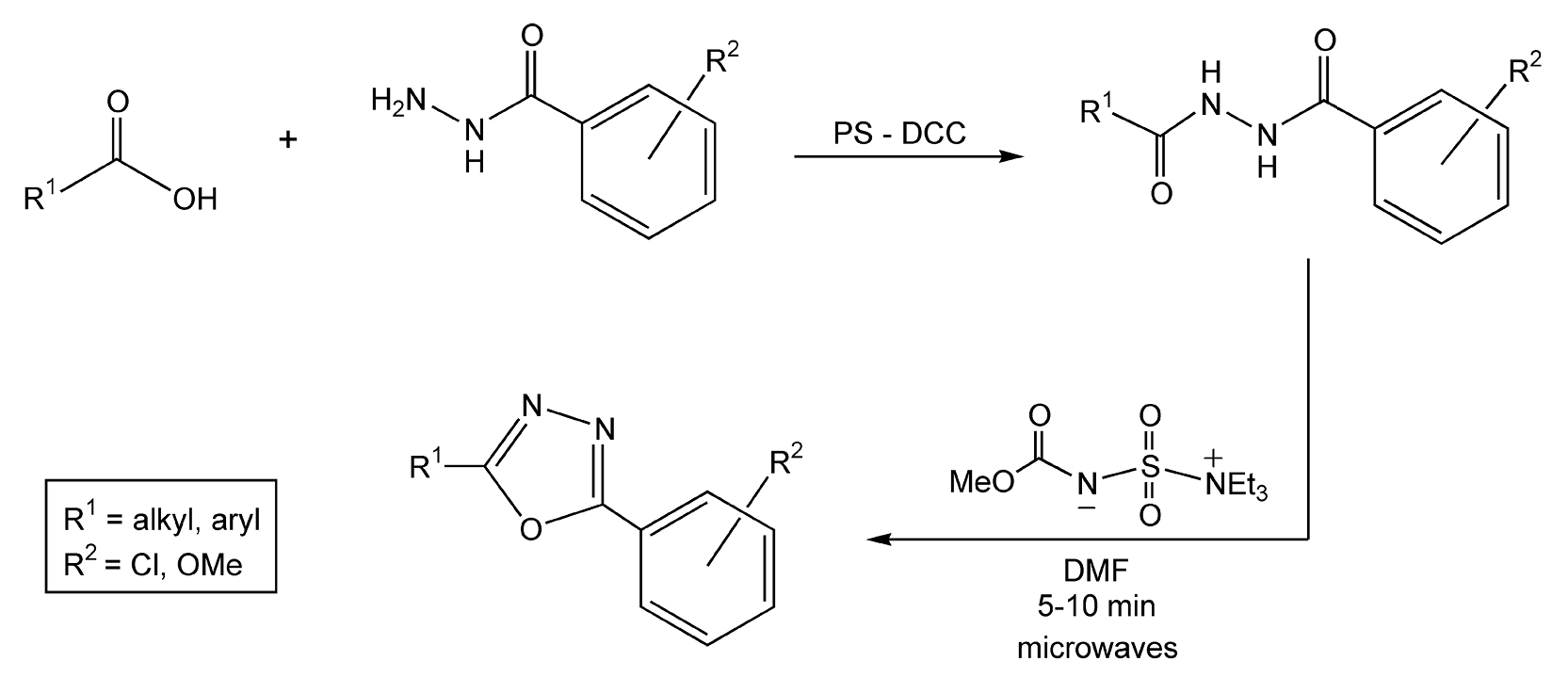
Scheme 63
Another organic transformation that benefits from the use of microwave irradiation is a 1,3-dipolar reaction.23,61-63,155,157,177,355-378 These types of reactions are cycloadditions, but they are being discussed in this section because they form heterocyclic moieties. One of the main reactants, which are generated in situ, is a 1,3-dipole. 1,3-Dipoles are heteroatom molecules that contain both a positive and a negative charge. Due to ionic conduction, these ionic species will directly interact with the microwave energy being applied. They readily react with a dipolarophile, which is usually an electrophilic alkene or alkyne, to form heterocyclic ring systems. Using conventional methods, these cycloadditions can take 1-2 days. In Scheme 64, nitrile oxides (X = O) and nitrile imines (X = NPh) are irradiated with microwaves to yield different heterocyclic compounds in only 30 minutes.355
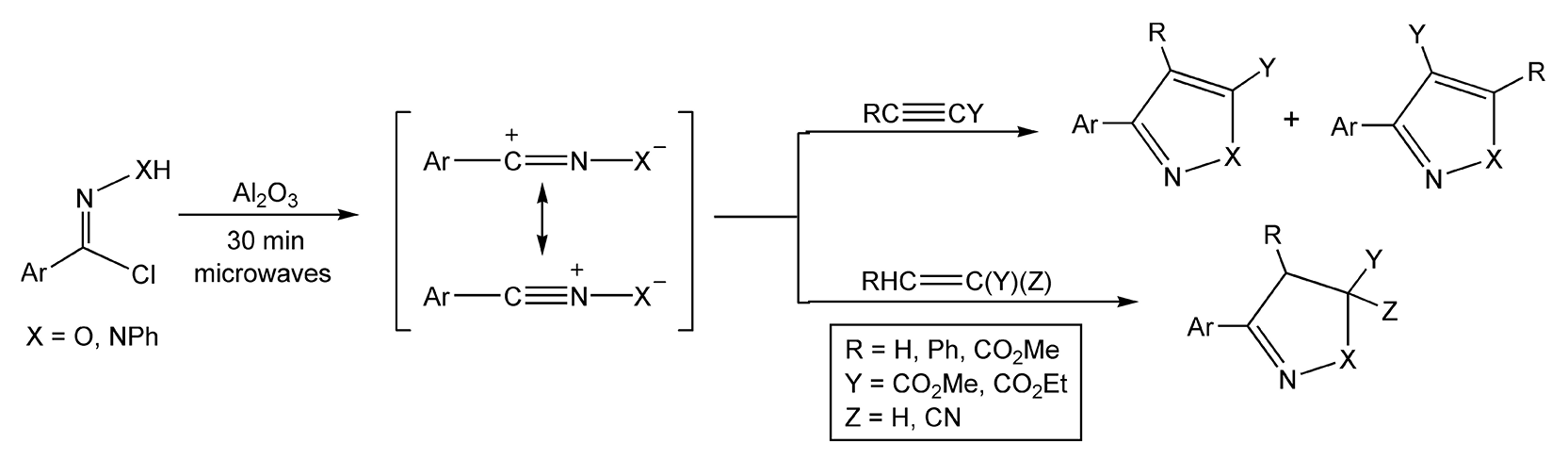
Scheme 64
Instruments
11. Bose, A.K.; Manhas, M.S.; Ghosh, M.; Shah, M.; Raju, V.S.; Bari, S.S.; Newaz, S.N.; Banik, B.K.; Chaudhary, A.G.; Barakat, K.J. “Microwave-induced organic reaction enhancement chemistry. 2. Simplified techniques.” J. Org. Chem. 1991, 56, pp. 6968-70.
47. Paul, S.; Gupta, M.; Gupta, R.; Loupy, A. “Microwave assisted solvent-free synthesis of pyrazolo[3,4-b]quinolines and pyrazolo[3,4-c]pyrazoles using p-TsOH.” Tetrahedron Lett. 2001, 42, pp. 3827-29.
49. Rodriguez, H.; Perez, R.; Suarez, M.; Lam, A.; Cabrales, N.; Loupy, A. “Alkylation of some pyrimidine and purine derivatives in the absence of solvent using microwave-assisted method.” Fifth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5) 2001, E0004 (www.mdpi.net).
57. Marrero-Terrero, A.L.; Loupy, A. “Synthesis of 2-oxazolines from carboxylic acids and α,α,α-tris(hydroxy-methyl)methylamine under microwaves in solvent-free conditions.” Synlett. 1996, 3, pp. 245-46.
58. Perez, E.; Sotelo, E.; Loupy, A.; Mocelo, R.; Suarez, M.; Perez, R.; Autie, M. “An easy and efficient microwave-assisted method to obtain 1-(4-bromophenacyl)azoles in dry media.” Heterocycles 1996, 47, pp. 539-43.
59. Suarez, M.; Loupy, A.; Perez, E.; Moran, L.; Gerona, G.; Morales, A.; Autie, M. “An efficient procedure to obtain hexahydroquinomeines and unsymmetrical 1,4-dihydropyridines using solid inorganic supports and microwave activation.” Heterocycl. Commun. 1996, 2, pp. 275-80.
64. Diaz-Ortiz, A.; de la Hoz, A.; Langa, F. “Microwave irradiation in solvent-free conditions: an eco-friendly methodology to prepare indazoles, pyrazolopyridines and bipyrazoles by cycloaddition reactions.” Green Chem. 2000, 2, pp. 165-72.
67. Balalaie, S.; Sharifi, A.; Ahangarian, B.; Kowsari, E. “Microwave enhanced synthesis of quinazolines in solvent-free condition.” Heterocycl. Commun. 2001, 7, pp. 337-40.
68. Balalaie, S.; Kowsari, E. “One-pot synthesis of N-substituted 4-aryl-1,4-dihydropyridines under solvent-free condition and microwave irradiation.” Fifth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5) 2001, E0026 (www.mdpi.net).
71. Balalaie, S.; Hashtroudi, M.S.; Sharifi, A. “Microwave-assisted synthesis of 1,3,5-trialkyltetrahydro-1,3,5-triazin-2(1H)-ones and a 4-oxooxadiazinane in dry media.” J. Chem. Res. (S) 1999, pp. 392-93.
96. Varma, R.S.; Saini, R.K. “Microwave-assisted isomerization of 2’-aminochalcones on clay: an easy route to 2-aryl-1,2,3,4-tetrahydro-4-quinolones.” Synlett. 1997, 87, pp. 857-58.
106. Kidwai, M.; Sapra, P. “An expeditious solventless synthesis of isoxazoles.” Org. Prep. Proced. Intl. 2001, 33, pp. 381-86.
107. Kidwai, M.; Sapra, P.; Misra, P.; Saxena, R.K.; Singh, M. “Microwave-assisted solid support synthesis of novel 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazepines as potent antimicrobial agents.” Bioorg. Med. Chem. 2001, 9, pp. 217-220.
109. Kidwai, M.; Sapra, P.; Bhushan, K.R.; Misra, P. “Microwave-assisted synthesis of novel 1,2,4-triazines in ‘dry media’.” Synth. Commun. 2001, 31, pp. 1639-45.
110. Kidwai, M., Bhushan, K.R.; Sapra, P.; Saxena, R.K.; Gupta, R. “Alumina-supported synthesis of antibacterial quinolines using microwaves.” Bioorg. Med. Chem. 2000, 8, pp. 69-72.
111. Kidwai, M.; Venkataramanan, R.; Kohli, S. “Alumina-supported synthesis of β-lactams using microwave.” Synth. Commun. 2000, 30, pp. 989-1002.
112. Kidwai, M.; Misra, P.; Dave, B.; Bhushan, K.R.; Saxena, R.K.; Singh, M. “Microwave-activated solid support synthesis of new antibacterial quinolones.” Monatsh. Chem. 2000, 131, pp. 1207-12.
113. Kidwai, M.; Misra, P.; Bhushan, K.R. “Microwave assisted synthesis of novel organomercurials in ‘dry media’.” Polyhedron 1999, 18, pp. 2641-43.
118. Xu, Q.; Chao, B.; Wang, Y.; Dittmer, D.C. “Tellurium in the “no-solvent” organic synthesis of allylic alcohols.” Tetrahedron 1997, 53, pp. 12131-146.
119. Loghmani-Khouzani, H.; Sadeghi, M.M.; Safari, J.; Minaeifar, A. “A novel method for the synthesis of 2-ketomethylquinolines under solvent-free conditions using microwave irradiation.” Tetrahedron Lett. 2001, 42, pp. 4363-64.
125. Pezet, F.; Sasaki, I.; Daran, J.C.; Hydrio, J.; Ait-Haddou, H.; Balavoine, G. “First example of supported microwave-assisted synthesis of new chiral bipyridines and a terpyridine - use in asymmetric cyclopropanation.” Eur. J. Inorg. Chem. 2001, pp. 2669-74.
130. Shanmugam, P.; Singh, P.R. “Montmorillonite K-10 clay-microwave assisted isomerisation of acetates of the Baylis-Hillman adducts: a facile method of stereoselective synthesis of (E)-trisubstituted alkenes.” Synlett. 2001, 8, pp. 1314-16.
131. Vanden Eynde, J.J.; Hecq, N.; Kappe, C.O.; Kataeva, O. “Microwave-mediated regioselective synthesis of novel pyrimido[1,2-a]pyrimidines under solvent-free conditions.” Tetrahedron 2001, 57, pp. 1785-91 and Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4) 2000, A0050 (www.mdpi.net).
133. Villemin, D.; Hammadi, M.; Martin, B. “Clay catalysis: condensation of orthoesters with o-substituted aminoaromatics into heterocycles.” Synth. Commun. 1996, 26, pp. 2895-99.
155. Kerneur, G.; Lerestif, J.M.; Bazureau, J.P.; Hamelin, J. “Convenient preparation of 4-alkylidene-lH-imidazol-5(4H)-one derivatives from imidate and aldehydes by a solvent-free cycloaddition under microwaves.” Synthesis 1997, 3, pp. 287-89.
156. Cherouvrier, J.R.; Bazureau, J.P. “A practical and stereo-selective route to 5-ylidene-3,5-dihydroimidazol-4-one derivatives using solvent-free conditions under focused microwave irradiations.” Fifth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5) 2001, E0012 (www.mdpi.net).
157. Fraga-Dubreuil, J.; Cherouvrier, J.R.; Bazureau, J.P. “Clean solvent-free dipolar cycloaddition reactions assisted by focused microwave irradiations for the synthesis of new ethyl 4-cyano-2-oxazoline-4-carboxylates.” Green Chem. 2000, 2, pp. 226-29.
169. Stefani, H.A.; Gatti, P.M. “3,4-Dihydropyrimidin-2(1H)-ones: fast synthesis under microwave irradiation in solvent-free conditions.” Synth. Commun. 2000, 30, pp. 2165-73.
177. Corsaro, A.; Chiacchio, U.; Librando, V.; Fisichella, S.; Pistara, V. “1,3-Dipolar cycloadditions of polycyclic aromatic hydrocarbons with nitrile oxides under microwave irradiation in the absence of solvent.” Heterocycles 1997, 45, pp. 1567-72.
238. Falsone, F.S.; Kappe, C.O. “The Biginelli dihydropyrimidone synthesis using polyphosphate ester as a mild and efficient cyclocondensation/dehydration reagent.” Ark. Org. Kemi. 2001, 2, pp. 1111-23.
239. Stadler, A.; Kappe, C.O. “Automated library generation using sequential microwave-assisted chemistry. Application toward the Biginelli multicomponent condensation.” J. Comb. Chem. 2001, 3, pp. 624-30.
240. Stadler, A.; Kappe, C.O. “Microwave-mediated Biginelli reactions revisited. On the nature of rate and yield enhancements.” J. Chem. Soc., Perkin Trans. 1 2000, pp. 1363-68.
241. Yadav, J.S.; Reddy, B.V.S.; Reddy, E.J.; Ramalingam, T. “Microwave-assisted synthesis of dihydropyrimidines: improved high yielding protocol for the Biginelli reaction.” J. Chem. Res. (S) 2000, pp. 354-55.
242. Kappe, C.O.; Kumar, D.; Varma, R.S. “Microwave-assisted high-speed parallel synthesis of 4-aryl-3,4-dihydropyrimidin-2(1H)-ones using solventless Biginelli condensation protocol.” Synthesis 1999, pp. 1799-803.
243. Rahmouni, M.; Derdour, A.; Bazureau, J.P.; Hamelin, J. “A new access to 2,3-dihydroimidazo[1,2c]pyrimidines.” Synth. Commun. 1996, 26, pp. 453-58.
244. Rahmouni, M.; Derdour, A.; Bazureau, J.P.; Hamelin, J. “A new route to pyrimido[1,6a]benzimidazoles: reactivity of activated 2-benzimidazoles with N-acyl imidates as β-dielectrophiles under microwave irradiation.” Tetrahedron Lett. 1994, 35, pp. 4563-64.
245. Kappe, C.O.; Shishkin, O.V.; Uray, G.; Verdino, P. “X-ray structure, conformational analysis, enantioseparation, and determination of absolute configuration of the mitotic kinesin Eg5 inhibitor monastrol.” Tetrahedron 2000, 56, pp. 1859-62.
246. Dandia, A.; Saha, M.; Taneja, H. “Synthesis of fluorinated ethyl-4-aryl-6-methyl-1,2,3,4-tetrahydropyrimidin-2-one/thione-5-carboxylates under microwave irradiation.” J. Fluorine Chem. 1998, 90, pp. 17-21.
247. Saloutin, V.I.; Bughart, Y.V.; Kuzueva, O.G.; Kappe, C.O.; Chupakhin, O.N. “Biginelli condensations of fluorinated 3-oxo esters and 1,3-diketones.” J. Fluorine Chem. 2000, 103, pp. 17-23.
248. Gupta, R.; Gupta, A.K.; Paul, S.; Kachroo, P.L. “Improved syntheses of some ethyl-4-aryl-6-methyl-1,2,3,4-tetrahydropyrimidin-2-one/thione-5-carboxylates by microwave irradiation.” Indian J. Chem. 1995, 34B, pp. 151-52.
249. Dave, C.G.; Shah, R.D. “Gould-Jacobs type of reaction in the synthesis of thieno[3,2e]pyrimido[1,2c]pyrimidines: a comparison of classical heating vs. solvent-free microwave irradiation.” Heterocycles 1999, 51, pp. 1819-26.
250. Cablewski, T.; Gurr, P.A.; Pajalic, P.J.; Strauss, C.R. “A solvent-free Jacobs-Gould reaction.” Green Chem. 2000, 1, pp. 25-28.
251. Meziane, M.A.A.; Rahmouni, M.; Bazureau, J.P.; Hamelin, J. “A new route to 1-oxo-1,2-dihydropyrimido[1,6a]benzimidazole-4-carboxylates from ethyl-2-(benzimidazol-2-yl)-3-(dimethylamino)acrylate using solvent-free conditions.” Synthesis 1998, 7, pp. 967-69.
252. Ferreira, V.F.; de Souza, M.C.B.V.; Cunha, A.C.; Pereira, L.O.R.; Ferreira, M.L.G. “Recent advances in the synthesis of pyrroles.” Org. Prep. Proced. Intl. 2001, 33, pp. 411-54.
253. Ranu, B.C.; Hajra, A. “Synthesis of alkyl-substituted pyrroles by three-component coupling of carbonyl compound, amine and nitro-alkane/alkene on a solid surface of silica gel/alumina under microwave irradiation.” Tetrahedron 2001, 57, pp. 4767-73.
254. Rao, H.S.P.; Jothilingam, S. “One-pot synthesis of pyrrole derivatives from (E)-1,4-diaryl-2-butene-1,4-diones.” Tetrahedron Lett. 2001, 42, pp. 6595-97.
255. Danks, T.N. “Microwave assisted synthesis of pyrroles.” Tetrahedron Lett. 1999, 40, pp. 3957-60.
256. Jolivet-Fouchet, S.; Hamelin, J.; Texier-Boullet, F.; Toupet, L.; Jacquault, P. “Novel pathway to 1-aminopyrroles and other nitrogen heterocycles from glyoxal monohydrazones and acylated active methylene compounds in solvent-free reactions under microwave irradiation.” Tetrahedron 1998, 54, pp. 4561-78.
257. Ranu, B.C.; Hajra, A.; Jana, U. “Microwave-assisted synthesis of substituted pyrroles by a three-component coupling of α,β-unsaturated carbonyl compounds, amines, and nitro alkanes on the surface of silica gel.” Synlett. 2000, 1, pp. 75-76.
258. Bakavoli, M.; Germaninejhad, H.; Rahimizadeh, M.; Ghassemzadeh, M.; Heravi, M.M. “Microwave assisted heterocyclization: a rapid and efficient synthesis of imidazo[4,5-b]pyridines.” Indian J. Heterocyclic Chem. 2001, 10, pp. 317-18.
259. Khadilkar, B.M.; Madyar, V.R. “Scaling up of dihydropyridine ester synthesis by using aqueous hydrotrope solutions in a continuous microwave reactor.” Org. Process Res. Dev. 2001, 5, pp. 452-55.
260. Ohberg, L.; Westman, J. “An efficient and fast procedure for the Hantzsch dihydropyridine synthesis under microwave conditions.” Synlett. 2001, pp. 1296-98.
261. Zhou, J.F.; Tu, S.J.; Feng, J.C. “One-step synthesis of pyridine derivatives from malononitrile with bisarylidenecycloalkanone under microwave irradiation.” J. Chem. Res. (S) 2001, pp. 268-69.
262. Sharma, U.; Ahmed, S.; Boruah, R.C. “A facile synthesis of annelated pyridines from β-formyl enamides under microwave irradiation.” Tetrahedron Lett. 2000, 41, pp. 3493-95.
263. Díaz-Ortiz, A.; Carrillo, J.R.; Cossío, F.P.; Gómez-Escalonilla, M.J.; de la Hoz, A.; Moreno, A.; Prieto, P. “Synthesis of pyrazolo[3,4-b]pyridines by cycloaddition reactions under microwave irradiation.” Tetrahedron 2000, 56, p. 1569.
264. Varma, R.S.; Kumar, D. “Microwave-accelerated three-component condensation reaction on clay: solvent-free synthesis of imidazo[1,2-a] annulated pyridines, pyrazines, and pyrimidines.” Tetrahedron Lett. 1999, 40, pp. 7665-69.
265. Vega, J.A.; Vaquero, J.J.; Alvarez-Builla, J.; Ezquerra, J.; Hamdouchi, C. “A new approach to the synthesis of 2-aminoimidazo[1,2-a]pyridine derivatives through microwave-assisted N-alkylation of 2-halopyridines.” Tetrahedron 1999, 55, pp. 2317-26.
266. Cotterill, I.C.; Usyatinsky, A.Y.; Arnold, J.M.; Clark, D.S.; Dordick, J.S.; Michels, P.C.; Khmelnitsky, Y.L. “Microwave assisted combinatorial chemistry: synthesis of substituted pyridines.” Tetrahedron Lett. 1998, 39, pp. 1117-20.
267. Khadilkar, B.M.; Gaikar, V.G.; Chitnavis, A.A. “Aqueous hydrotrope solution as a safer medium for microwave enhanced Hantzsch dihydropyridine ester synthesis.” Tetrahedron Lett. 1996, 37, p. 1719.
268. Villemin, D.; Vlieghe, X. “Thiation under microwave irradiation. II: Synthesis of sulfur heterocycles.” Sulfur Lett. 1998, 21, pp. 199-203.
269. Alajarin, R.; Jordan, P.; Vaquero, J.J.; Alvarez-Builla, J. “Synthesis of unsymmetrically substituted 1,4-dihydropyridines and analogous calcium antagonists by microwave heating.” Synthesis 1995, 4, pp. 389-91.
270. Zhang, Y.W.; Shen, Z.X.; Pan, B.; Lu, X.H.; Chen, M.H. “Research on the synthesis of 1,4-dihydropyridines under microwave.” Synth. Commun. 1995, 25, pp. 857-62.
271. Khadilkar, B.M.; Gaikar, V.G.; Chitnavis, A.A. “Aqueous hydrotrope solution as a safer medium for microwave enhanced Hantzsch dihydropyridine ester synthesis.” Tetrahedron Lett. 1995, 36, pp. 8083-86.
272. Khadilkar, B.M.; Chitnavis, A.A. “Rate enhancement in the synthesis of some 4-aryl-1,4-dihydropyridines using methyl-3-aminocrotonate, under microwave irradiation.” Indian J. Chem. 1995, 34B, pp. 652-53.
273. Penieres, G.; Garcia, O.; Franco, K.; Hernandez, O.; Alvarez, C. “A modification to the Hantzsch method to obtain pyridines in a one pot reaction: use of bentonitic clay in a dry medium.” Heterocycl. Commun. 1996, 2, pp. 359-60.
274. Paul, S.; Gupta, R.; Loupy, A. “Improved synthesis of 2-amino-3-cyanopyridines in solvent free conditions under microwave irradiation.” J. Chem. Res. (S) 1998, pp. 330-31.
275. Linder, M.R.; Podlech, J. “Synthesis of β-lactams from diazoketones and imines: the use of microwave irradiation.” Org. Lett. 2001, 3, pp. 1849-51.
276. Kidwai, M.; Venkataramanan, R.; Kohli, S. “Alumina-supported synthesis of β-lactams using microwave.” Synth. Commun. 2000, 30, pp. 989-1002.
277. Kidwai, M.; Sapra, P.; Bhushan, K.R.; Saxena, R.K.; Gupta, R.; Singh, M. “Microwave-assisted stereoselective synthesis and antibacterial activity of new fluoroquinolinyl-β-lactam derivatives.” Monatsh. Chem. 2000, 131, pp. 85-90.
278. Manhas, M.S.; Banik, B.K.; Mathur, A.; Vincent, J.E.; Bose, A.K. “Vinyl-β-lactams as efficient synthons. Eco-friendly approaches via microwave assisted reactions.” Tetrahedron 2000, 56, pp. 5587-601.
279. Bose, A.K.; Banik, B.K.; Mathur, C.; Wagle, D.R.; Manhas, M.S. “Polyhydroxy amino acid derivatives via β-lactams using enantiospecific approaches and microwave techniques.” Tetrahedron 2000, 56, pp. 5603-19.
280. Martelli, G.; Spunta, G.; Panunzio, M. “Microwave-assisted solvent-free organic reactions: synthesis of β-lactams from 1,3-azadienes.” Tetrahedron Lett. 1998, 39, pp. 6257-60.
281. Kidwai, M.; Kumar, K.; Kumar, P. “Microwave induced stereoselective synthesis and antibacterial activity of β-lactams.” J. Indian Chem. Soc. 1998, 75, pp. 102-03.
282. Banik, B.K.; Jayaraman, M.; Srirajan, V.; Manhas, M.S.; Bose, A.K. “Rapid synthesis of β-lactams as intermediates for natural products via eco-friendly reactions.” J. Indian Chem. Soc. 1997, 74, pp. 943-47.
283. Banik, B.K.; Manhas, M.S.; Robb, E.W.; Bose, A.K. “Environmentally benign chemistry: microwave-induced stereocontrolled synthesis of β-lactam synthons.” Heterocycles 1997, 44, pp. 405-16.
284. Khajavi, M.S.; Sefidkon, F.; Hosseini, S.S.S. “Reaction of imines with trichloroacetic esters or anhydride promoted by iron carbonyl or microwave irradiation. Preparation of 3,3-dichloro-β-lactams.” J. Chem. Res. (S) 1998, pp. 724-25.
285. Bose, A.K.; Banik, B.K.; Manhas, M.S. “Studies on lactams. Part 98. Stereocontrol of β-lactam formation using microwave irradiation.” Tetrahedron Lett. 1995, 36, pp. 213-16.
286. Kidwai, M.; Kumar, R.; Kohli, S. “Microwave-induced synthesis of nitrogen-mustard derivatives.” Indian J. Chem. 1999, 38B, pp. 1132-35.
287. Azizian, J.; Soozangarzadeh, S.; Jadidi, K. “Microwave-induced one-pot synthesis of some new spiro[3H-indole-3,5’(4’H)-[1,2,4]-triazoline]-2-ones.” Synth. Commun. 2001, 31, pp. 1069-73.
288. Dandia, A.; Sachdeva, H.; Singh, R. “Improved synthesis of 3-spiroindolines in dry media under microwave irradiation.” Synth. Commun., 2001, 31, pp. 1879-92.
289. Dandia, A.; Singh, R.; Sachdeva, H.; Arya, K. “Microwave assisted one pot synthesis of a series of trifluoromethyl-substituted spiro[indole-triazoles].” J. Fluorine Chem. 2001, 111, pp. 61-67.
290. Kidwai, M.; Misra, P. “Microwave-induced “solvent-free” novel technique for the synthesis of spiro[indole-pyrazole/isoxazole/pyrimidine] derivatives.” Oxidation Commun. 2001, 24, pp. 287-90.
291. Dandia, A.; Sachdeva, H.; Devi, R. “Montmorillonite catalysed synthesis of novel spiro[3H-indole-3,3’-[3H-1,2,41]triazol]-2(1H)-ones in dry media under microwave irradiation.” J. Chem. Res. (S) 2000, pp. 272-75
292. Gribble, G.W. “Recent developments in indole ring synthesis-methodology and applications.” J. Chem. Soc., Perkin Trans. 1 2000, pp. 1045-75.
293. Dandia, A.; Saha, M.; Taneja, H. “Improved one-pot synthesis of 3-spiroindolines under microwave irradiation.” Phosphorus Sulfur Silicon Relat. Elem. 1998, 139, pp. 77-85.
294. Sridar, V. “Microwave radiation as a catalyst for chemical reactions.” Curr. Sci. 1998, 74, pp. 446-50.
295. Sridar, V. “Rate acceleration of Fischer-indole cyclization by microwave irradiation.” Indian J. Chem., Sec. B 1996, 35, pp. 737-38.
296. Abramovitch, R.A. “Applications of microwave energy in organic chemistry. A review.” Org. Prep. Proced. Intl. 1991, 23, pp. 683-711.
297. Lipifiska, T.; Guibe-Jampel, E.; Petit, A.; Loupy, A. “2-(2-Pyridyl)indole derivatives preparation via Fischer reaction on montmorillonite K10/zinc chloride under microwave irradiation.” Synth. Commun. 1999, 29, pp. 1349-54.
298. Jnaneshwara, G.K.; Bedekar, A.V.; Deshpande, V.H. “Microwave assisted preparation of isatins and synthesis of (+)-convolutamydine-A.” Synth. Commun. 1999, 29, pp. 3627-33.
300. Ranu, B.C.; Hajra, A.; Jana, U. “Microwave-assisted simple synthesis of quinolines from anilines and alkyl vinyl ketones on the surface of silica gel in the presence of indium(III) chloride.” Tetrahedron Lett. 2000, 41, pp. 531-33.
301. Sabitha, G.; Babu, R.S.; Reddy, B.V.S.; Yadav, J.S. “Microwave assisted Friedlaender condensation catalyzed by clay.” Synth. Commun. 1999, 29, pp. 4303-08.
302. Ahluwalia, V.K.; Goyal, B.; Das, U. “One-pot syntheses of 5-oxo-1,4,5,6,7,8-hexahydroquinolines and pyrimido[4,5b]quinolines using microwave irradiation and ultrasound.” J. Chem. Res. (S) 1997, p. 266.
303. Huang, Z.Z.; Wu, L.L.; Huang, X. “Facile synthesis of 2-alkyl and 2-aryl-4-quinolones using microwave irradiation.” Chin. J. Org. Chem 2000, 20, pp. 88-90.
304. Besson, T.; Dozias, M.J.; Guillard, J.; Jacquault, P.; Legoy, M.D.; Rees, C.W. “Expeditious routes to 4-alkoxyquinazoline-2-carbonitriles and thiocarbamates via N-arylimino-1,2,3-dithiazoles using microwave irradiation.” Tetrahedron 1998, 54, pp. 6475-84.
305. Besson, T.; Guillard, J.; Rees, C.W. “Multistep synthesis of thiazoloquinazolines under microwave irradiation in solution.” Tetrahedron Lett. 2000, 41, pp. 1027-30.
306. Seijas, J.A.; Vazquez-Tato, M.P.; Martinez, M.M. “Microwave enhanced synthesis of 4-aminoquinazolines.” Tetrahedron Lett. 2000, 41, pp. 2215-17.
307. Rad-Moghadam, K.; Khajavi, M.S. “One-pot synthesis of substituted quinazoline-4(3H)-ones under microwave irradiation.” J. Chem. Res. (S) 1998, pp. 702-03.
308. Khajavi, M.S.; Rad-Moghadam, K.; Hazarkhani, H. “A facile synthesis of 6-substituted benzimidazo[1,2c]quinazolines under microwave irradiation.” Synth. Commun. 1999, 29, pp. 2617-24.
309. Besson, T.; Rees, C.W. “New route to 4-alkoxyquinazoline-2-carbonitriles.” J. Chem. Soc., Perkin Trans. 1 1996, pp. 2857-60.
310. Bougrin, K.; Loupy, A.; Petit, A.; Daou, B.; Soufiaoui, M. “Novel synthesis of 2-trifluoromethylarylimidazoles on montmorillonite K-10 in a ‘dry medium’ under microwave irradiation.” Tetrahedron 2001, 57, pp. 163-68.
311. Fresneda, P.M.; Molina, P.; Sanz, M.A. “Microwave-assisted regioselective synthesis of 2,4-disubstituted imidazoles: Nortopsentin D synthesized by minimal effort.” Synlett. 2001, pp. 218-21.
312. Rostamizadeh, S.; Derafshian, E. “A simple route to the preparation of benzimidazoles and benzoxazoles.” J. Chem. Res. (S) 2001, pp. 227-28.
313. Balalaie, S.; Arabanian, A. “One-pot synthesis of tetrasubstituted imidazoles catalyzed by zeolite HY and silica gel under microwave irradiation.” Green Chem. 2000, 2, pp. 274-76.
314. Balalaie, S.; Arabanian, A.; Hashtroudi, M.S. “Zeolite HY and silica gel as new and efficient heterogenous catalysts for the synthesis of triarylimidazoles under microwave irradiation.” Monatsh. Chem. 2000, 131, pp. 945-48.
315. Hashtroudi, M.S.; Nia, S.S.; Asadollahi, H.; Balalaie, S. “Microwave promoted synthesis of benzimidazole derivatives in solvent free condition.” Indian J. Heterocycl. Chem. 2000, 9, pp. 307-308.
316. Usyatinsky, A.Y.; Khmelnitsky, Y.L. “Microwave-assisted synthesis of substituted imidazoles on a solid support under solvent-free conditions.” Tetrahedron Lett. 2000, 41, pp. 5031-34.
317. Glas, H.; Thiel, W.R. “Microwave assisted synthesis of chiral imidazolyl and pyrazolyl alcohols.” Tetrahedron Lett. 1998, 39, pp. 5509-10.
318. Bougrin, K.; Soufiaoui, M. “Novel syntheses of arylimidazoles by microwave irradiation in dry medium.” Tetrahedron Lett. 1995, 36, pp. 3683-86.
319. Khajavi, M.S.; Hajihadi, M.; Naderi, R. “Synthesis of heterocyclic compounds from o-substituted anilines under microwave irradiation.” J. Chem. Res. (S) 1996, pp. 92-93.
320. Khajavi, M.S.; Hajihadi, M.; Nikpour, F. “Various syntheses of benzimidazolin-2-ones and benzimidazoline-2-thiones under microwave irradiation.” J. Chem. Res. (S) 1996, pp. 94-95.
321. Aghapoor, K.; Heravi, M.M.; Nooshabadi, M.A. “Synthesis of benzimidazoles in a solvent-free reaction under microwave activation.” Indian J. Chem. 1998, 37B, p. 84.
322. Ben-Alloum, A.; Bakkas, S.; Soufiaoui, M. “Benzimadazoles: oxidative heterocyclization by nitrobenzene or DMSO on silica and under microwave and UV irradiation.” Tetrahedron Lett. 1998, 39, pp. 4481-84.
323. Bougrin, K.; Loupy, A.; Soufiaoui, M. “Three new routes for synthesis of 1,3-azole derivatives using microwaves.” Tetrahedron 1998, 54, pp. 8055-64.
324. Kamal, A.; Reddy, B.S.N.; Reddy, G.S.K. “Microwave assisted synthesis of pyrrolo[2,1c]1,4”-benzodiazepine-5,11-diones.” Synlett. 1999, 8, pp. 1251-52.
325. Jolivet-Fouchet, S.; Fabis, F.; Bovy, P.; Ochsenbein, P.; Rault, S. “Novel rearrangement of pyrrolo[2,1c]1,4”-benzodiazepines into pyrrolo[2,1b]quinazolinones, analogs of alkaloid vasicinone.” Heterocycles 1999, 51, pp. 1257-73.
326. Mekheimer, R.; Shaker, R.M.; Sadek, K.U.; Otto, H.H. “A novel synthesis of benzo[g”]imidazo[1,2a]pyridines: the reactivity of arylidene-1H-benzimidazole-2-acetonitrile with electron poor olefins and dimethylacetylene dicarboxylate under microwave irradiation.” Heterocycl. Commun. 1997, 3, pp. 217-21.
327. Reddy, A.C.S.; Rao, P.S.; Venkataratnam, R.V. “Fluoro organics: Facile syntheses of novel 2- or 4-trifluoromethyl-1H-arylo-1,5-diazepines, oxazepines, thiazepines, 2-(1,1,1-trifluoro-acetonyl) imidazoles, oxazoles, and thiazoles.” Tetrahedron 1997, 53, pp. 5847-54.
328. Oussaid, B.; Moeini, L.; Martin, B.; Villemin, D.; Garrigues, B. “Improved synthesis of oxadiazoles under microwave irradiation.” Synth. Commun. 1995, 25, pp. 1415-19.
329. Brain, C.T.; Paul, J.M.; Loong, Y.; Oakley, P.J. “Novel procedure for the synthesis of 1,3,4-oxadiazoles from 1,2-diacylhydrazines using polymer-supported Burgess reagent under microwave conditions.” Tetrahedron Lett. 1999, 40, pp. 3275-78.
330. Brain, C.T.; Paul, J.M. “Rapid synthesis of oxazoles under microwave conditions.” Synlett. 1999, pp. 1642-44.
331. Lee, J.C.; Song, I.G. “Mercury(II) p-toluenesulfonate mediated synthesis of oxazoles under microwave irradiation.” Tetrahedron Lett. 2000, 41, pp. 5891-94.
332. Oussaid, B.; Berlan, J.; Soufiaoui, M.; Garrigues, B. “Improved synthesis of oxazoline under microwave irradiation.” Synth. Commun. 1995, 25, pp. 659-65.
333. Clarke, D.S.; Wood, R. “A facile one stage synthesis of oxazolines under microwave irradiation.” Synth. Commun. 1996, 26, pp. 1335-40.
334. Gupta, R.; Paul, S.; Kamotra, P.; Gupta, A.K. “Rapid synthesis of S-triazolo[3,4b]-1,3,4-thiadiazoles and quinolines under microwave irradiation.” Indian J. Heterocycl. Chem. 1997, 7, pp. 155-56.
335. Kidwai, M.; Kumar, P. “Microwave-induced syntheses of 6-(substituted aryl)-3-[(5-methyl-1,3,4-thiadiazol-2-yl-sulfanyl)methyl]-1,2,4-triazolo[3,4b]-1,3,4-thiadiazoles.” J. Chem. Res. (S) 1996, pp. 254-55.
336. Kidwai, M.; Bhushan, K.R. “A rapid one-pot synthesis of 5-substituted-2-mercapto-1,3,4-thiadiazoles using microwaves.” Indian J. Chem. 1998, 37B, pp. 427-28.
337. Gupta, R.; Paul, S.; Gupta, A.K.; Kachroo, P.L. “Improved syntheses of some substituted 5,6-dihydro-S-triazolo[3,4b]-1,3,4-thiadiazoles in a microwave oven.” Indian J. Chem. 1994, 33B, pp. 888-91.
338. Azizian, J.; Morady, A.V.; Jadidi, K.; Mehrdad, M.; Sarrafi, Y. “Microwave-induced one-pot synthesis of some new spiro(indoline-3,2’-thiazolidine)-2,4’-(1H)-diones and bis[(spiro)indoline-3,2’-thiazolidine]-2,4’-(1H)-diones.” Synth. Commun. 2000, 30, pp. 537-42.
339. Ahluwalia, V.K.; Sharma, P.; Aggarwal, R. “Synthesis of 3-(1,3-diaryl-4,6-dioxo-2-thioxoperhydropyrimidin-5-yl)-2H-[1,4]benzothiazines.” J. Chem. Res. (S) 1997, pp. 16-17.
340. Ben-Alloum, A.; Bakkas, S.; Soufiaoui, M. “New synthesis pathway of 2-arylbenzothiazoles: transfer of electrons activated by microwaves.” Tetrahedron Lett. 1997, 38

