Electrophilic substitutions
Electrophilic substitutions also include a wide variety of different synthetic reactions. The majority of these reactions involve aromatic rings because most substitutions at an aliphatic carbon are nucleophilic. The converse is true with aromatic systems. They are more attracted to positively charged species because of their high electron density. Microwave irradiation has been used in examinations of Friedel-Crafts alkylations and acylations223,521-525, sulfonylations75,381,526,527, and deuterium-labeling528 of aromatic rings.
Friedel-Crafts alkylations and acetylations are probably the most well known electrophilic aromatic substitutions. In these reactions, a proton directly attached to the aromatic ring is replaced with an alkyl or acetyl group. Lewis acids are necessary to promote the formation of a cationic intermediate, which the aromatic ring attacks. Reaction times vary with aromatic ring activity when using conductive heating. Electron-donating groups (EDGs) provide faster reactions, whereas rings with electron-withdrawing groups (EWGs) react much slower. Reactions that are heated with microwaves proceed in five minutes or less, regardless of substituents. Scheme 87 shows an unusual reaction between mesitylene and formaldehyde.223 The carbocation that results is responsible for the Friedel-Crafts alkylation of another equivalent of mesitylene. This reaction proceeds in only four minutes with a 75% yield. Scheme 88 shows an intramolecular acylation on Bentonite (Al2O3•4SiO2•H2O, montmorillonite) that yields anthraquinone in five minutes.522
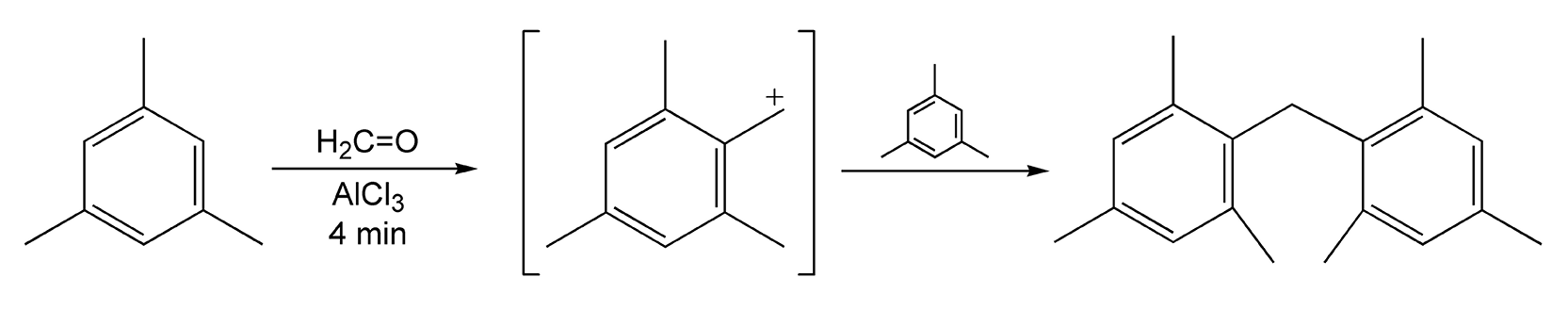
Scheme 87
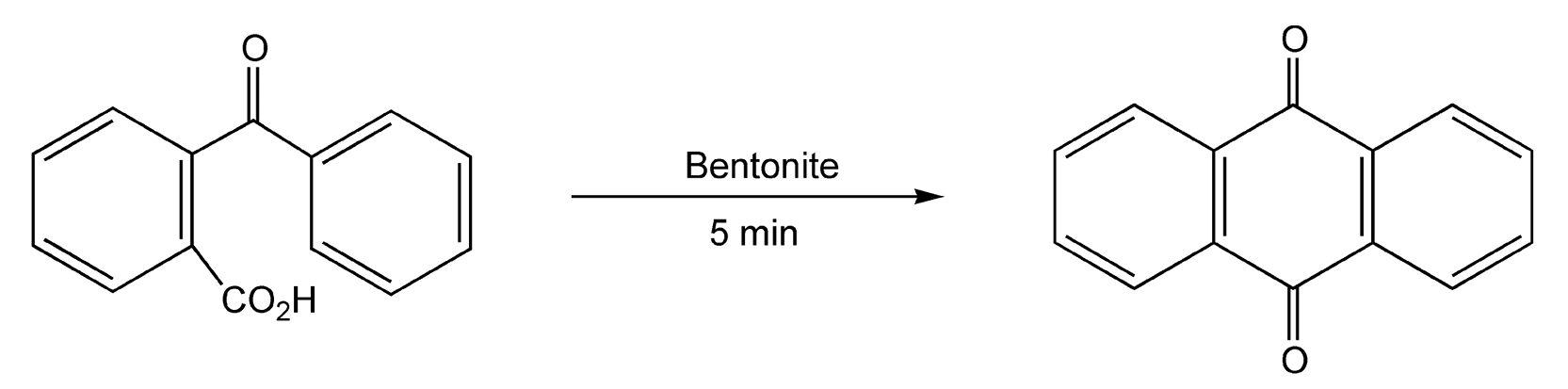
Scheme 88
Aromatic sulfonylation reactions are analogous to Friedel-Craft acylations. Generally, an aryl sulfonic acid chloride, coupled with a Lewis acid catalyst, reacts with an aromatic system to form a diaryl sulfone. The reaction can also be extended to the synthesis of alkyl aryl sulfones, from alkyl sulfonyl fluorides, and sulfonic acids, from sulfuric acid. With thermal conditions, these reactions usually require a stoichiometric amount of expensive catalyst and/or prolonged heating times. Marquie et al. have thoroughly examined electrophilic acylation and sulfonylation reactions under microwave irradiation.523-526 They have synthesized diaryl sulfones, using a catalytic amount of inexpensive FeCl3, in only five minutes with moderate to high product yields (Scheme 89).
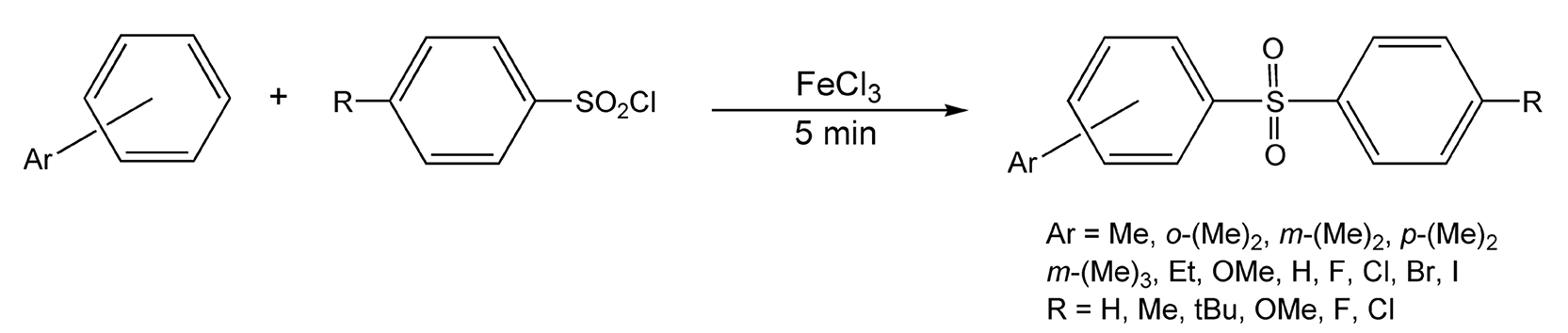
Scheme 89
The sulfonylation of naphthalene is a very tricky transformation, regardless of reaction conditions. Both regioisomers, α- and β-substituted, are usually formed despite the fact that the β-substituted isomer is thermodynamically more stable. Interestingly, with temperature-controlled microwave heating for three minutes, α-naphthalenesulfonic acid is predominately formed in a 19:1 ratio (Scheme 90).381
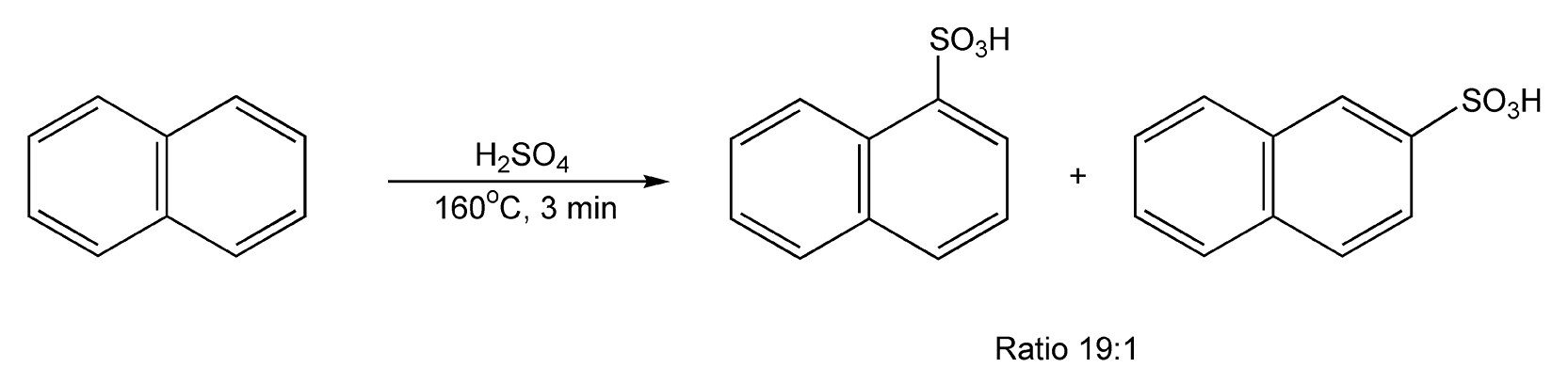
Scheme 90
Dehalogenation reactions are an effective and widely used method for deuterium-labeling (or tritium) of aromatic rings. Traditionally, labeling is achieved with either D2 or T2 gases. Both have solubility problems in organic solvents, and the storage of radioactive tritium waste is becoming an increasingly serious issue. Jones and co-workers have modified these labeling procedures by replacing D2 and T2 gases with labeled formates.528 Scheme 91 exhibits a dehalogenation reaction on halogen substituted benzamide compounds. Utilizing deuterium labeled potassium formate, palladium(II) acetate, and either DMSO or ethanol as a solvent, coupled with microwave irradiation for 20 seconds, these reactions yielded 94% labeled product.
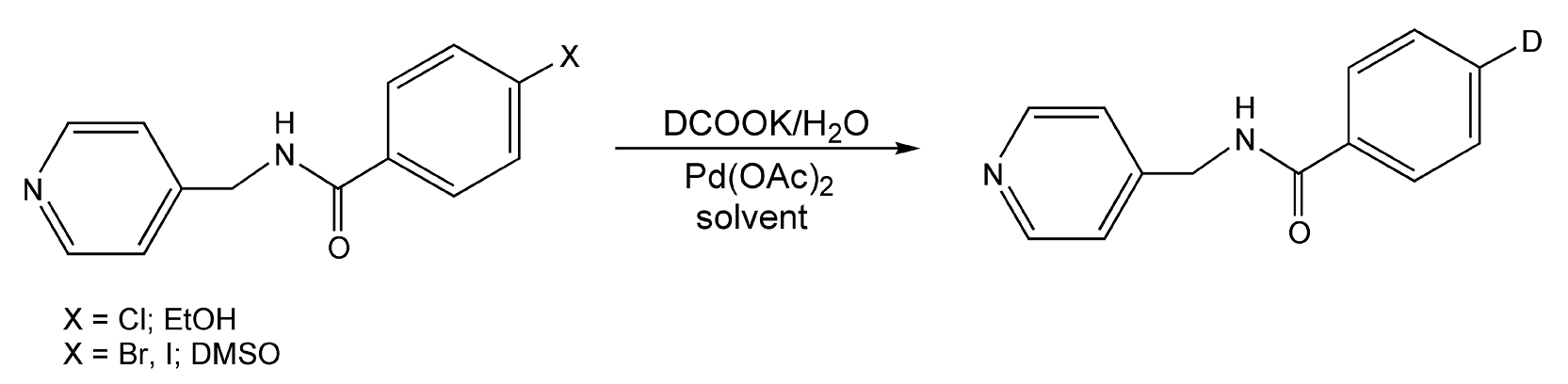
Scheme 91
I. Rearrangements
[3,3]-Sigmatropic rearrangements are important pericyclic reactions (concerted bond-making and -breaking). Two very important [3,3]-sigmatropic rearrangements are the Claisen and the Cope. In one type of Claisen rearrangement, an aryl vinyl ether rearranges to either an ortho-Claisen product or a para-Claisen product. In a Cope rearrangement, the products result from the rearrangement of a 1,5-hexadiene. Traditional methods for these transformations usually require very harsh reaction conditions, and in some cases, products will not form if the bulk temperature is less than 200 °C. Utilization of microwaves decreases reaction times from days to minutes.10,223,529,530 Scheme 92 shows an ortho-Claisen rearrangement in which both the reaction time and product yield were enhanced.
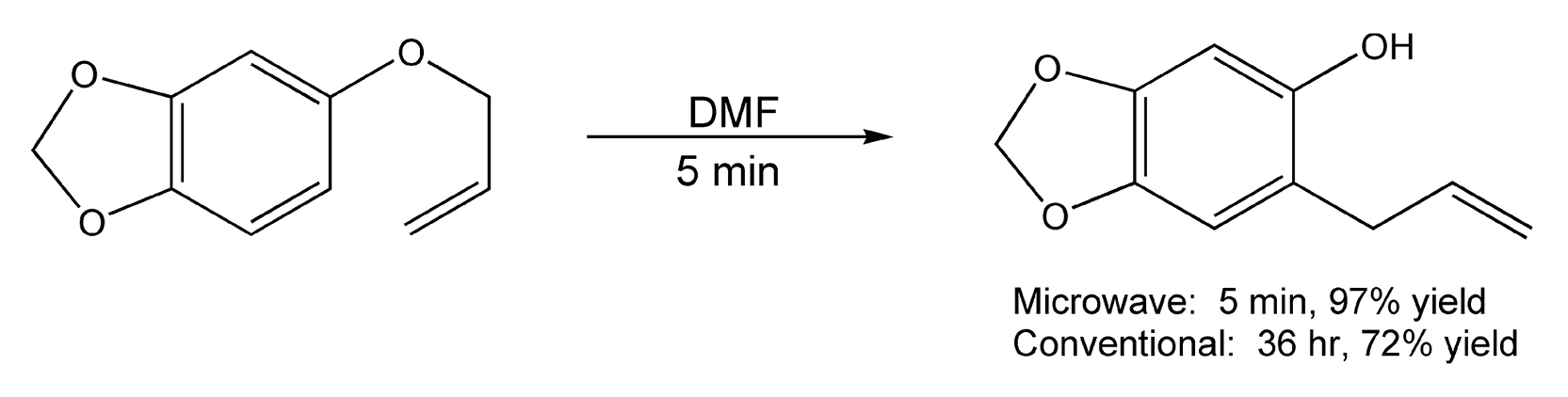
Scheme 92
In a para-Claisen rearrangement, an ortho-Claisen rearrangement is followed by a Cope rearrangement and tautomerization. In the example shown in Scheme 93, both the ortho-Claisen and the Cope are reversible transformations, but once tautomerization occurs to form the aromatic ring, the yield of the para product increases. Conventional methods usually require days of heating, but with microwave irradiation, the product is obtained in 20 minutes.10,223
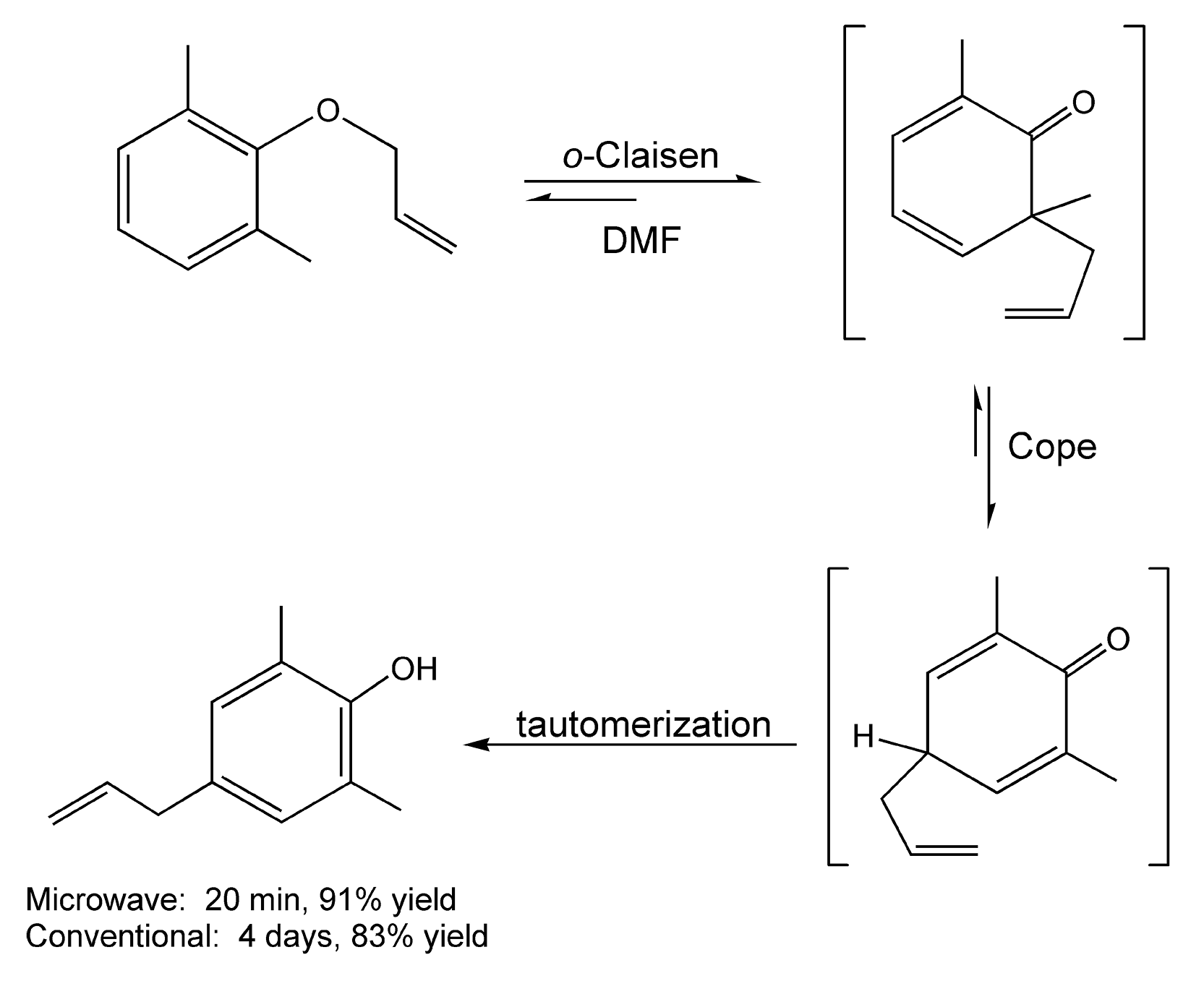
Scheme 93
The Fries rearrangement transforms an acyloxybenzene to an acylphenol. Acylphenols are important versatile organic intermediates that are used in agrochemical and pharmaceutical drug design. These reactions usually require stoichiometric amounts of Lewis acids and very long reflux times. In addition, they produce ortho/para mixtures. Moghaddam and co-workers have developed microwave-enhanced Fries rearrangements in dry media with 95% ortho-substituted products resulting (Scheme 94).531 Incidentally, when cinnamyl esters of phenols were used, conjugate addition followed the rearrangement to yield flavanone derivatives (Scheme 95).531
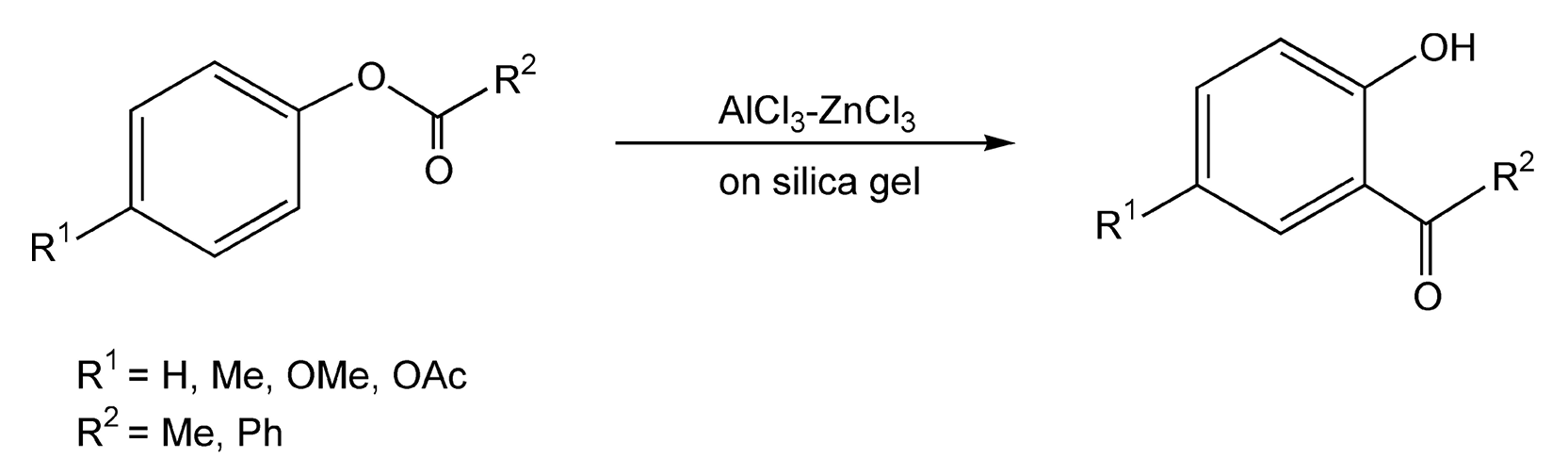
Scheme 94
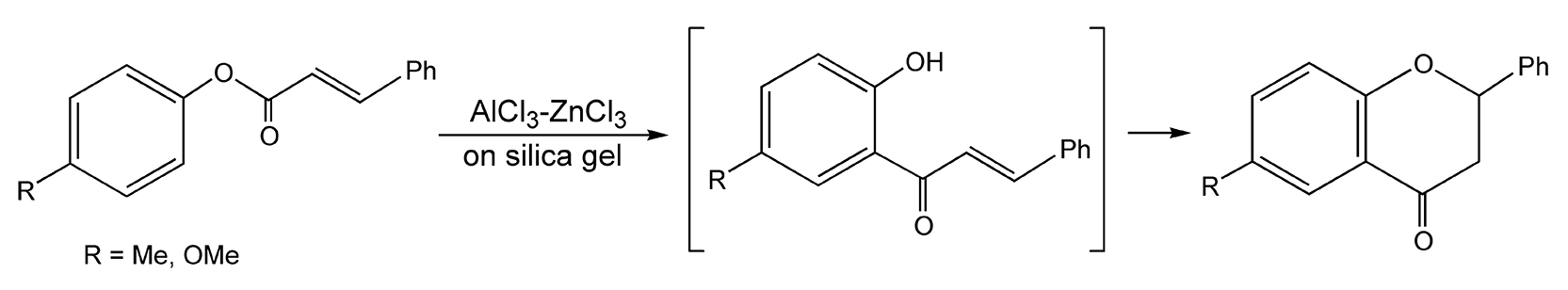
Scheme 95
Moghaddam et al. have also developed a thia-Fries rearrangement where aryl sulfonates rearrange to phenolic sulfones (Scheme 96).535
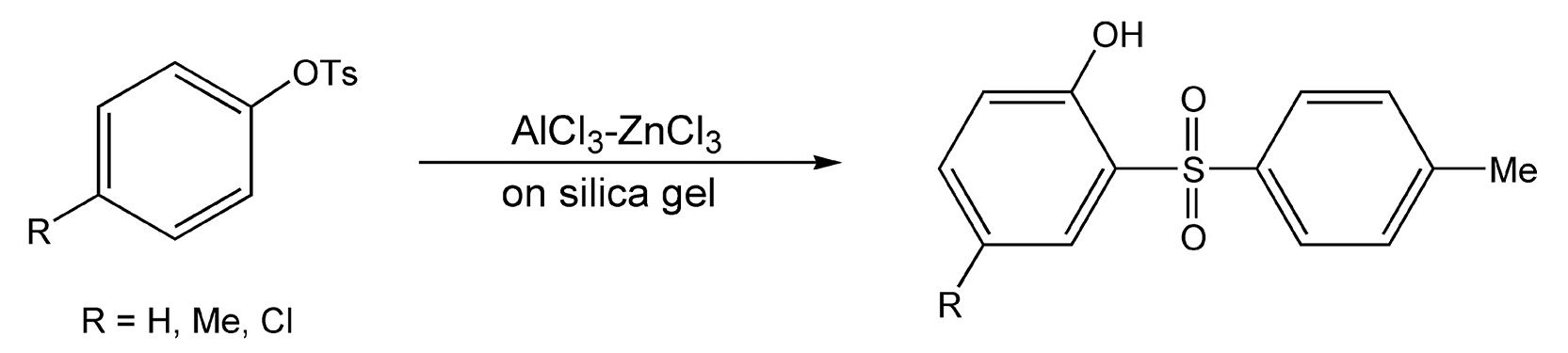
Scheme 96
Instruments
10. Majetich, G.; Hicks, R. “Applications of microwave-accelerated organic synthesis.” Radiat. Phys. Chem. 1995, 45, pp. 567-79.
75. Hajipour, A.R.; Mallakpour, S.E.; Imanzadeh, G. “An efficient and novel method for the synthesis of aromatic sulfones under solvent-free conditions.” Indian J. Chem. Sec. B 2001, 40, pp. 237-39.
223. Majetich, G.; Hicks, R. “The use of microwave heating to promote organic reactions.” J. Microwave Power Electromagnetic Energy 1995, 30, pp. 27-45.
381. Abramovitch, R.A.; Abramovitch, D.A.; Iyanar, K.; Tamareselvy, K. “Application of microwave energy to organic synthesis: improved technology.” Tetrahedron Lett. 1991, 32, pp. 5251-54.
521. Banik, B.K.; Becker, F.F. “Synthesis, electrophilic substitution, and structure-activity relationship studies of polycyclic aromatic compounds towards the development of anticancer agents.” Curr. Med. Chem. 2001, 8, pp. 1513-33.
522. Bram, G.; Loupy, A.; Majdoub, M.; Petit, A. “Anthraquinone microwave-induced synthesis in dry media in domestic ovens.” Chem. Ind. (London) 1991, pp. 396-97.
523. Marquie, J.; Salmoria, G.; Poux, M.; Laporterie, A.; Dubac, J.; Roques, N. “Acylation and related reactions under microwaves. 5. Development to large laboratory scale with a continuous-flow process.” Ind. Eng. Chem. Res. 2001, 40, pp. 4485-90.
524. Marquie, J.; Laporte, C.; Laporterie, A.; Dubac, J.; Desmurs, J.R.; Roques, N. “Acylation reactions under microwaves. 3. Aroylation of benzene and its slightly activated or deactivated derivatives.” Ind. Eng. Chem. Res. 2000, 39, pp. 1124-31.
525. Laporte, C.; Marquie, J.; Laporterie, A.; Desmurs, J.R.; Dubac, J. “Acylation reactions under microwaves. II. Acylation of aromatic ethers.” C.R. Acad. Sci., Ser. IIc: Chim. 1999, 2, pp. 455-65.
526. Marquie, J.; Laporterie, A.; Dubac, J.; Roques, N.; Desmurs, J.R. “Acylation and related reactions under microwaves. 4. Sulfonylation reactions of aromatics.” J. Org. Chem. 2001, 66, pp. 421-25.
527. Hajipour, A.R.; Mallakpour, S.E.; Imanzadeh, G. “An efficient and novel method for the synthesis of aromatic sulfones under solvent-free conditions.” Indian J. Chem. Sec. B 2001, 40, pp. 237-39.
528. Jones, J.R.; Lockley, W.J.S.; Lu, S.Y.; Thompson, S.P. “Microwave-enhanced aromatic dehalogenation studies: A rapid deuterium-labeling procedure.” Tetrahedron Lett. 2001, 42, pp. 331-32.
529. Srikrishna, A.; Nagaraju, S.; Kondaiah, P. “Application of microwave heating technique for rapid synthesis of γ,β-unsaturated esters.” Tetrahedron 1995, 51, pp. 1809-16.
530. Giraud, L.; Huber, V.; Jenny, T. “2,2-Divinyladamantane: a new substrate for the modification of silicon surfaces.” Tetrahedron 1998, 54, pp. 11899-906.
531. Moghaddam, F.M.; Ghaffarzadeh, M.; Abdi-Oskoui, S.H. “Tandem Fries reaction-conjugate addition under microwave irradiation in dry media; one-pot synthesis to flavanones.” J. Chem. Res. (S) 1999, pp. 574-75.
535. Moghaddam, F.M.; Dakamin, M.G. “Thia-Fries rearrangement of aryl sulfonates in dry media under microwave activation.” Tetrahedron Lett. 2000, 41, pp. 3479-81.

