Nucleophilic additions and substitutions
Nucleophilic addition and substitution reactions, both aromatic and aliphatic, encompass a large number of synthetic transformations. Microwave irradiation has been used extensively to enhance nucleophilic aromatic substitutions40,110,379-387, Michael additions148,192,388-402, Mitsunobu reactions403, hydroacylations404, N-acylations41,42,90,405-422, acetylations422-424, carbon382,389,425-439 and heteroatom alkylations (N66,172,265,317,421,439-464,465-469, 53-54,134,179,351,380,425,464,470-485), Williamson etherifications4,10,11,223,486-491, esterifications56,423,492-506, transesterifications41,158,507,508, halogenations10,181,223,510-518, and 18F-radiolabeling519,520.
Nucleophilic aromatic substitution (SNAr) reactions play an important role in drug discovery. A large number of drug compounds contain multiple aromatic rings. SNAr allows an organic chemist a facile route to changing substituents on the ring systems. Classically, SNAr requires long reaction times and high temperatures and provide low to moderate product yields. Scheme 65 shows two different substitutions on 1-chloro-4-nitrobenzene. Route A shows a substitution to an amine with ammonia and copper(I) oxide.381 With microwave irradiation, this transformation is successfully completed in one hour with a 93% product yield. Likewise, in route B, replacing the chlorine substituent with an ethoxy group forms an ether quantitatively in only two minutes.382
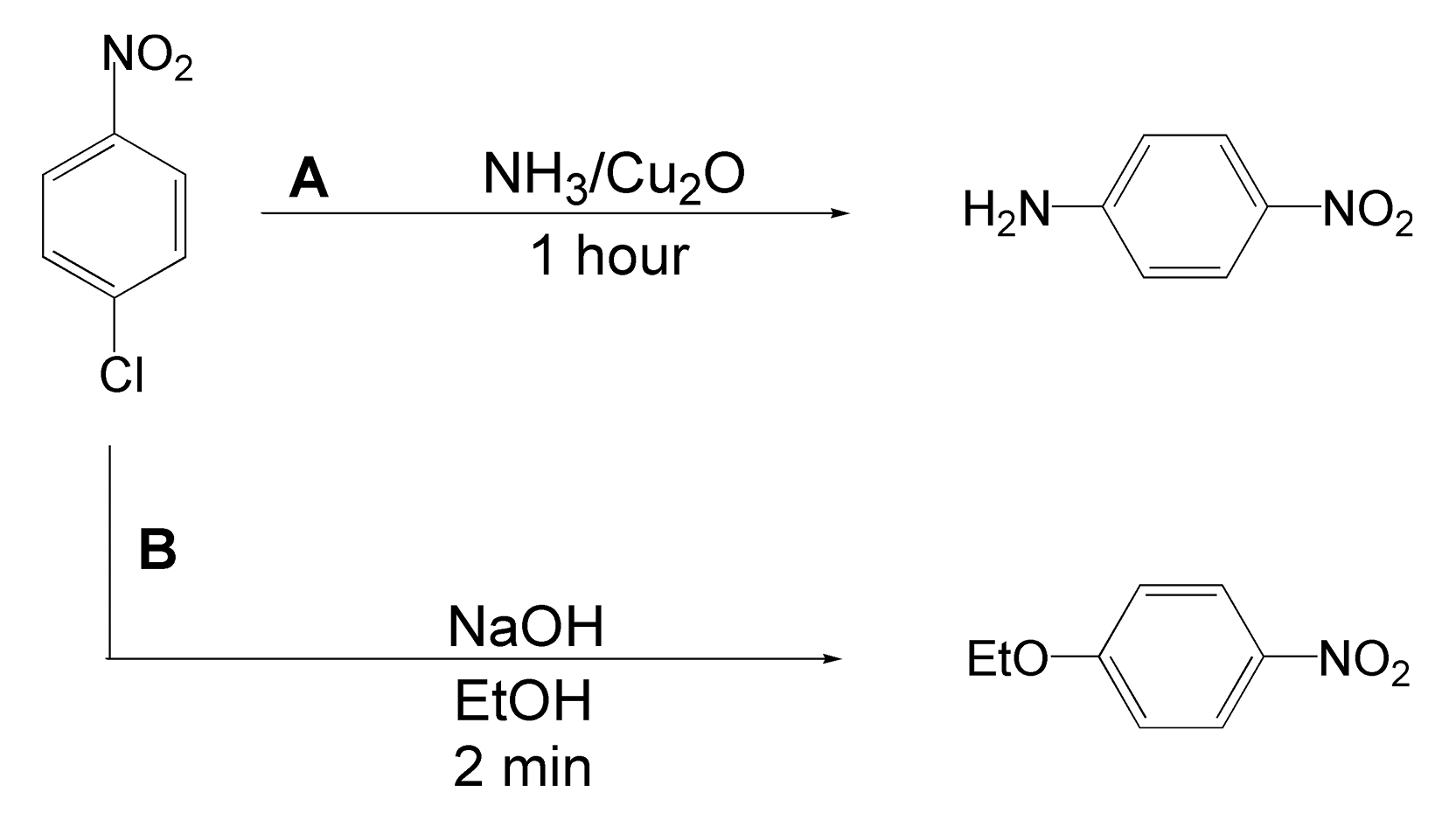
Scheme 65
Scheme 66 exhibits a small library of heterocyclic compounds that were synthesized using SNAr.354 Starting from one common aromatic scaffold; different amines were added, individually, to yield a small family of eight compounds. Using microwave instrumentation, this entire library was achieved in less than 90 minutes, whereas with conventional methods, this could take many days to complete. Additionally, the yields of this reaction greatly increased from as high as 60% with conventional heating to quantitative yields with microwave irradiation.
The Michael reaction forms the basis for many synthetic transformations. It involves a conjugate 1,4-addition of a nucleophile to an α,β-unsaturated ketone, aldehyde, amide, nitrile, nitride, sulfoxide, or sulfone. Scheme 67 shows an example of a Michael addition reaction between two indoles.388 Bis(indole) molecules have recently been isolated from sponges and are known bioactive metabolites. In this particular reaction, both the nitrovinylindole and alkylindole are adsorbed on silica gel and then subjected to microwave irradiation for 7-10 minutes. Using conventional methods, these additions proceeded in considerably longer reaction times, 8-14 hours.
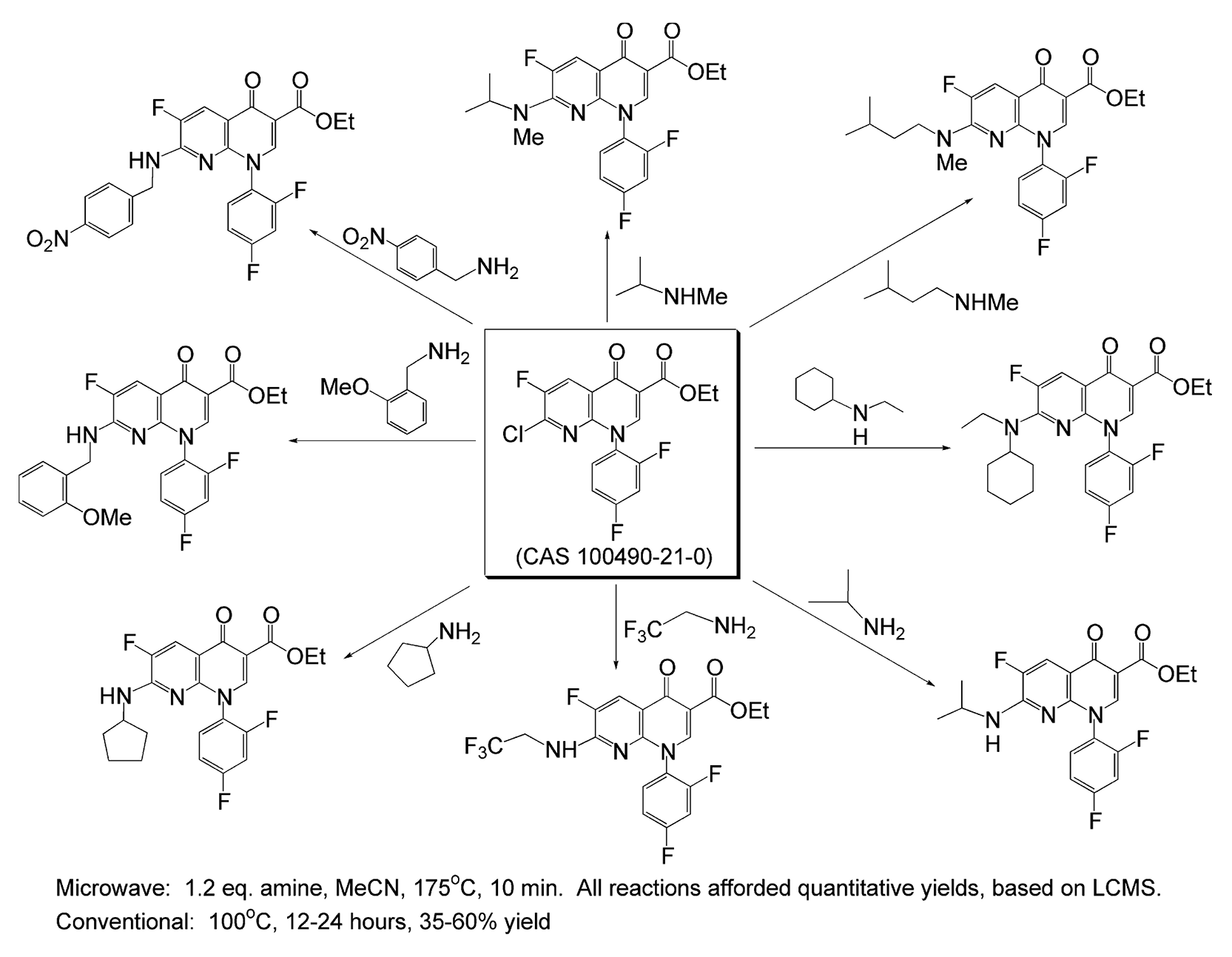
Scheme 66
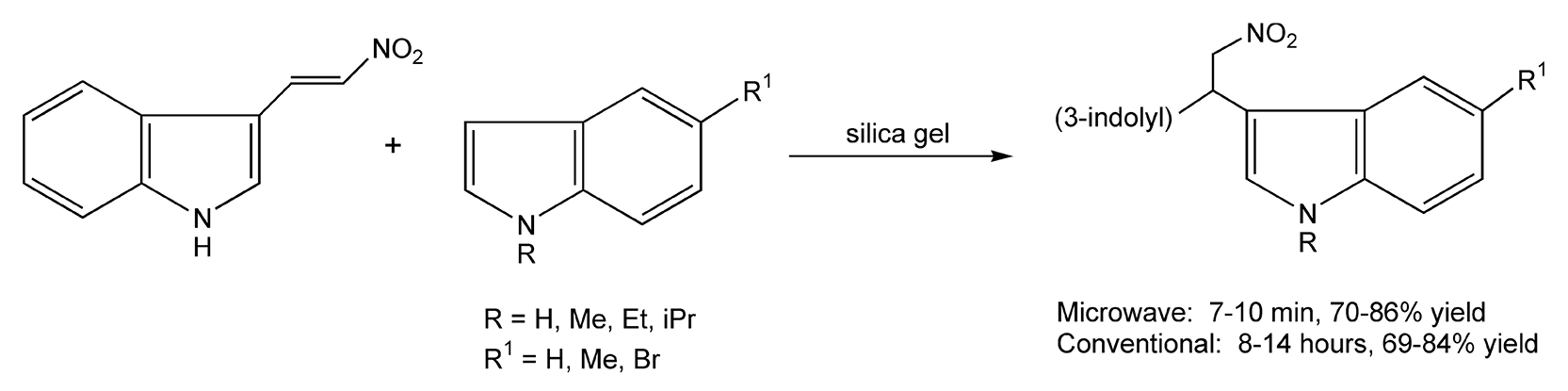
Scheme 67
Mitsunobu reactions are powerful synthetic transformations that can invert stereochemical configurations. Microwave irradiation has been used to enhance these conversions, as classical methods usually require high temperatures and long reaction times. Scheme 68 exhibits the acetylation of (S)-sulcatol via microwave enhanced Mitsunobu conditions (triphenylphosphine/diisopropyl azodicarboxylate) with acetic acid followed by lithium aluminum hydride (LAH) reduction to (R)-sulcatol.403
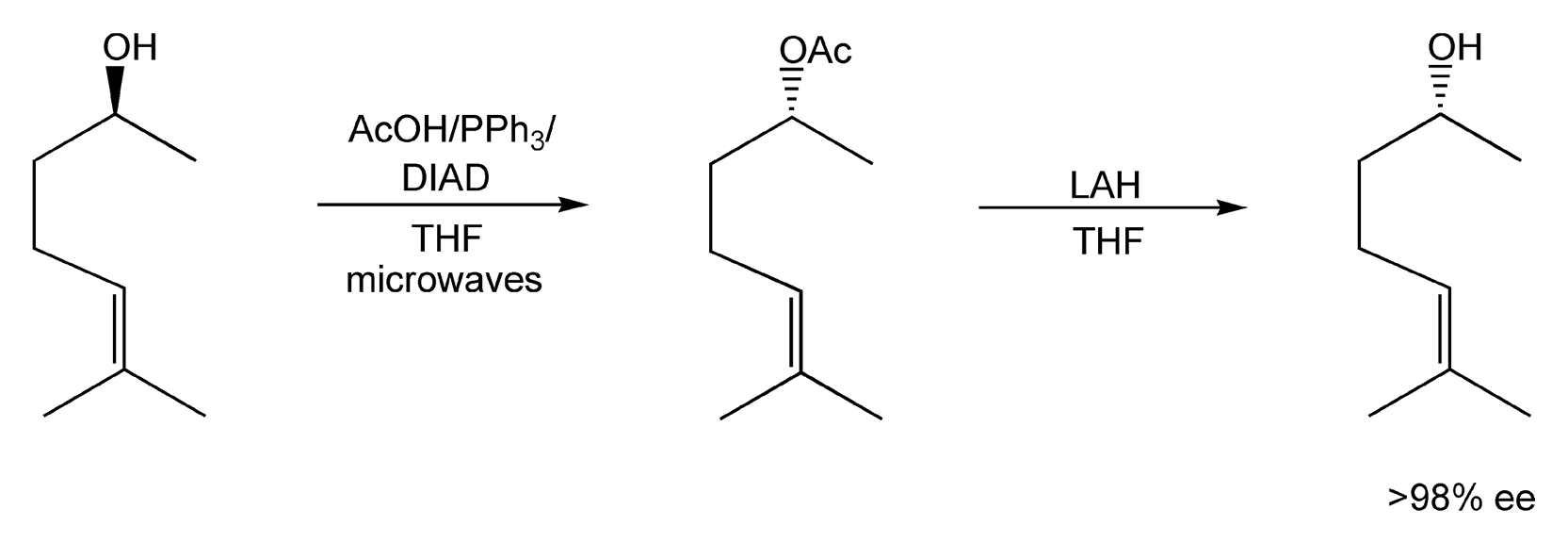
Scheme 68
Acylations [-C(O)R] and acetylations [-C(O)Me] are useful synthetic methods for obtaining ketones, amides, and enol esters. In hydroacylation reactions, aldehydes and olefins yield ketones via C–H bond activation by transition metals. Scheme 69 shows an efficient synthesis to ketones utilizing both Wilkinson’s rhodium(I) catalyst and 2-amino-3-picoline.5 The aldimine intermediate undergoes cyclometallation with the rhodium catalyst, which is then followed by alkene coordination. Alkene insertion followed by reductive elimination yields a ketone.404 Thermal methods can take 24 hours, but those reaction times have been reduced to four hours with a benzoic acid catalyst. With microwave heating, the reaction proceeds in ten minutes with moderate to high product yields.
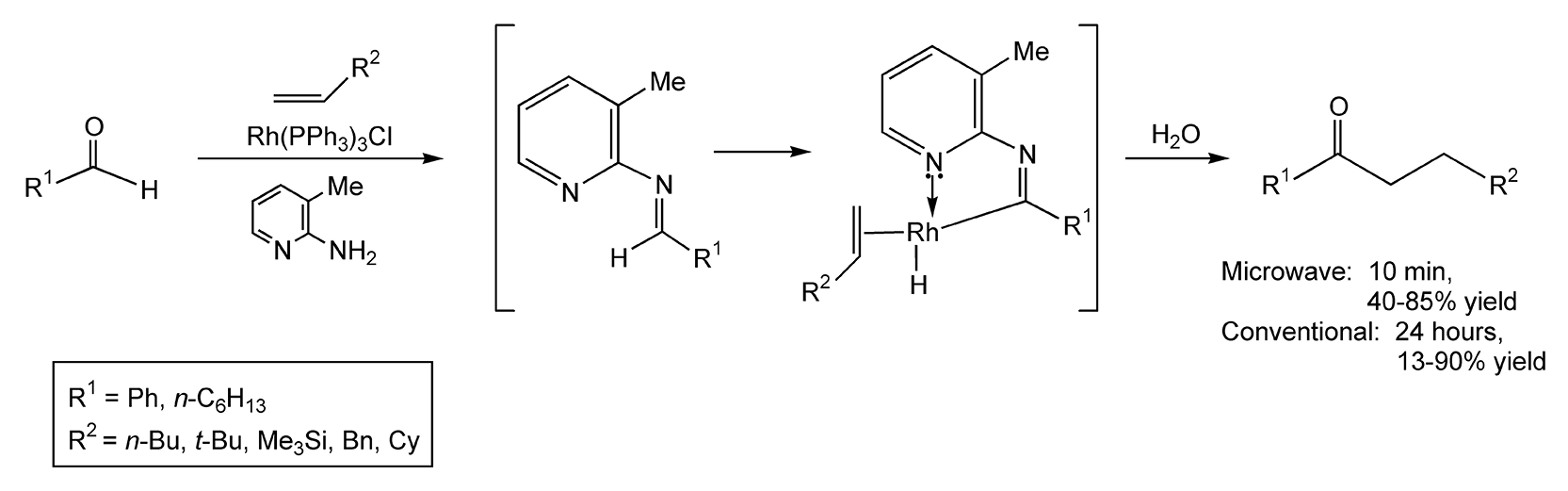
Scheme 69
Conversion of amines to amides is the most widely used protection method for amino groups. N-acylation of amines to maleimides is useful and can be enhanced with microwave heating (Scheme 70).405-410 Trifluoroacetylation is quite convenient in organic synthesis because of its facile cleavage. This acetylation is usually achieved with trifluoroacetic anhydride, but having trifluoroacetic acid as a byproduct causes one to look for alternative methods. The use of TiO(CF3CO2) provides a solution, as titanium oxide and water are the only byproducts. The use of this reagent on both primary and secondary amines, coupled with 5-10 minutes of microwave irradiation, gives trifluoroacetamides in excellent yields (Schemes 71 and 72).422 Use of conventional heating with the same reagents and reaction conditions takes at least 48 hours.
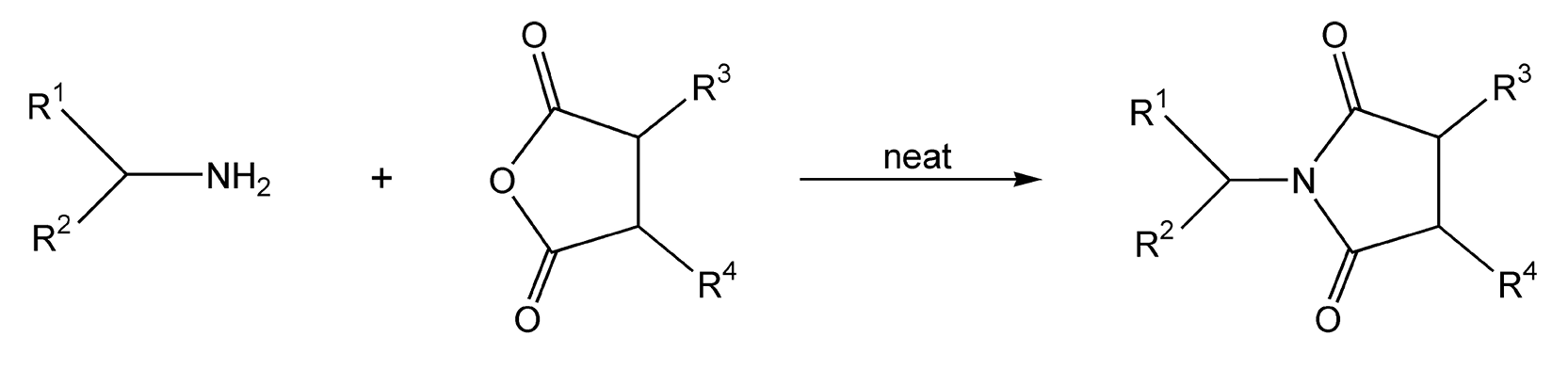
Scheme 70
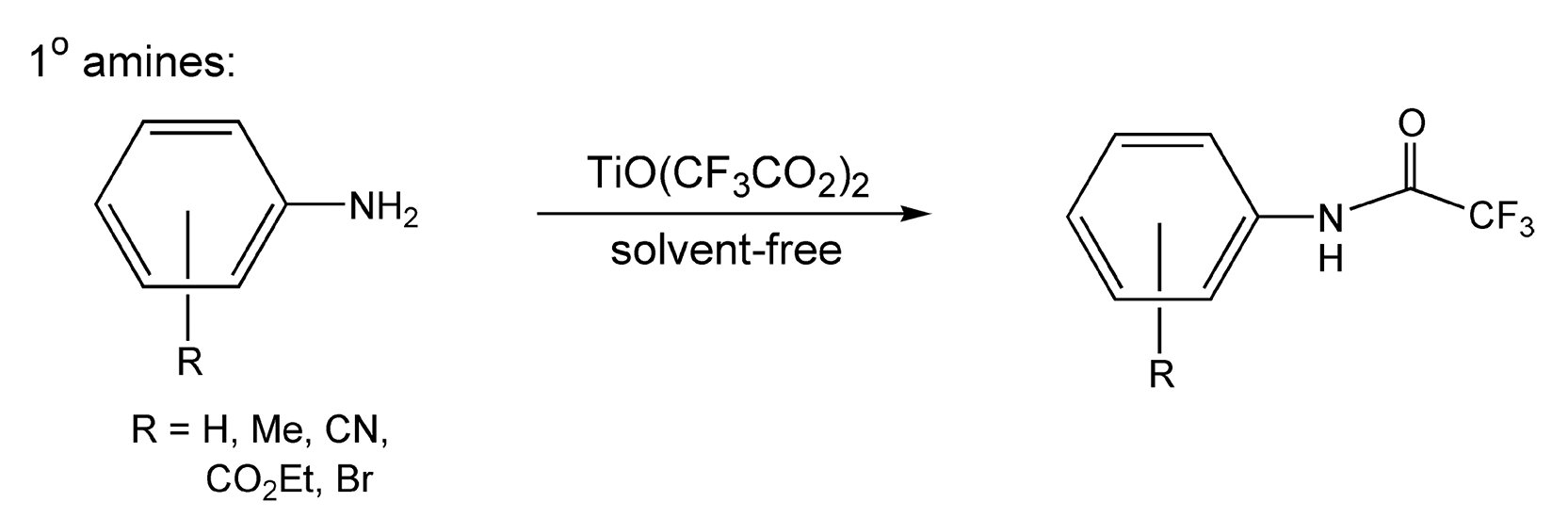
Scheme 71
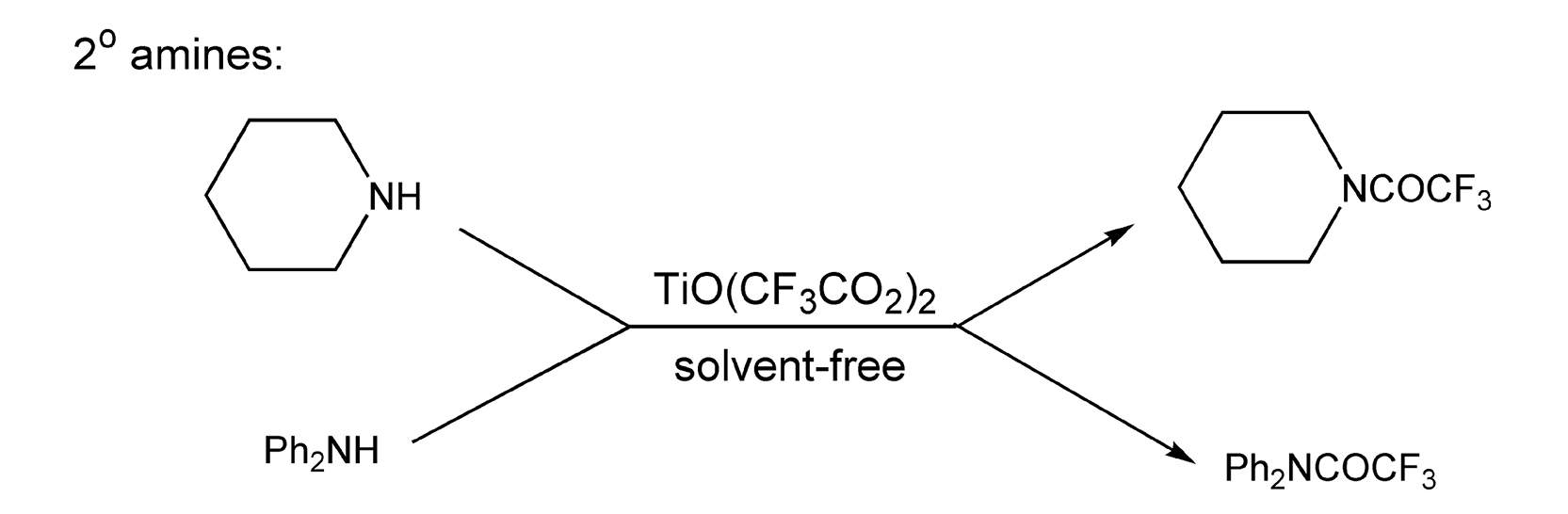
Scheme 72
Enol-acetylation of ketones is a valuable transformation in organic chemistry. The enol ethers that are formed are used extensively as intermediates in synthetic routes. Despite their popular usage, preparation methods are limited. A common procedure involves acetic anhydride with a basic or acid catalyst. These catalysts are very strong and can cause sensitive compounds to decompose. Scheme 73 shows a mild procedure that selectively acetylates six-membered cyclic ketones.423 With conductive heating, the cyclohexanone derivatives were refluxed in THF with acetic anhydride and iodine for 8 hours and gave very low product yields. Alternatively, quantitative yields were produced with microwave irradiation in only five to ten minutes.
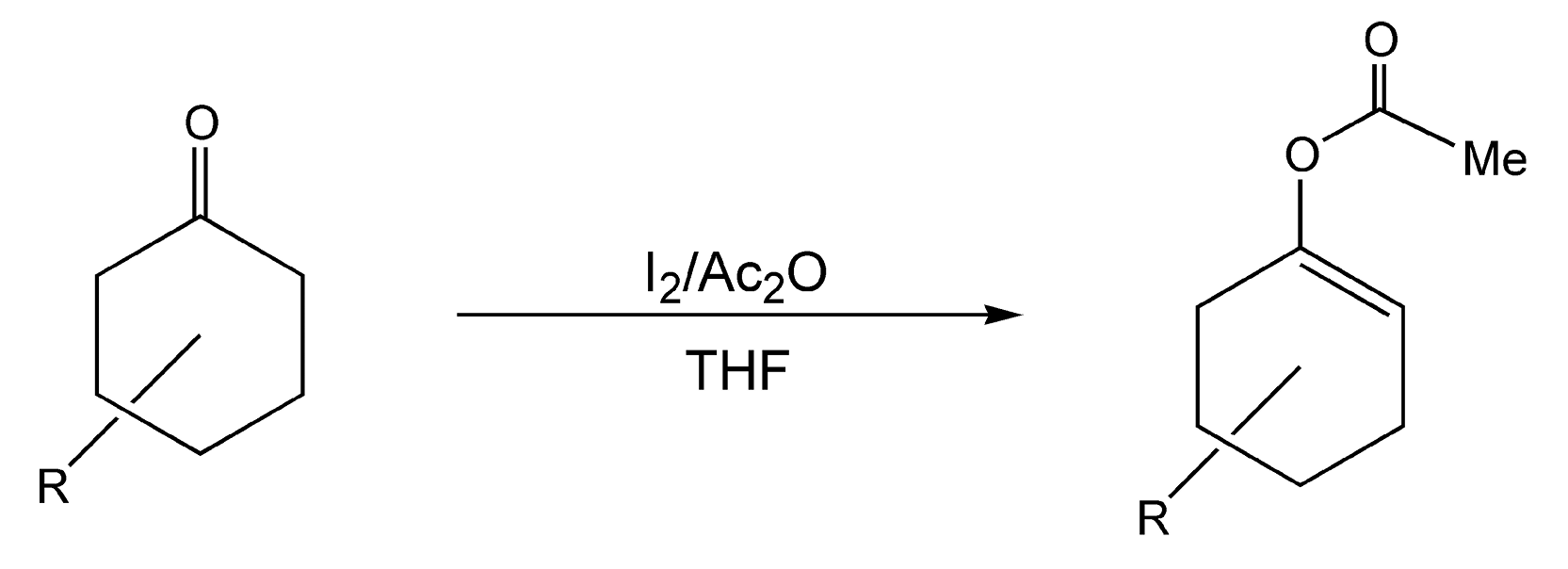
Scheme 73
Carbon-alkylations are also important reactions in organic chemistry. The most common and well-known method is by deprotonating a carbon that is adjacent to an electron-withdrawing group (e.g. enolate formation). Scheme 74 shows alkyl addition to a substituted acetate.382 With microwave heating, this reaction proceeded in 3 minutes with high product yields. Another example of C-alkylation is the addition of a 2-nitropropane anion (A) with a heterocyclic electrophile (B) (Scheme 75). The solvent conditions determine whether the final product is C or D, which is formed from subsequent elimination of nitrous acid from C.426
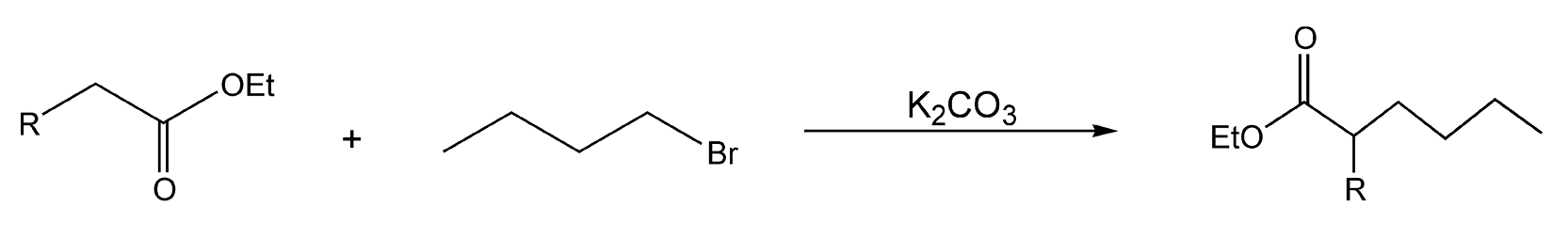
Scheme 74
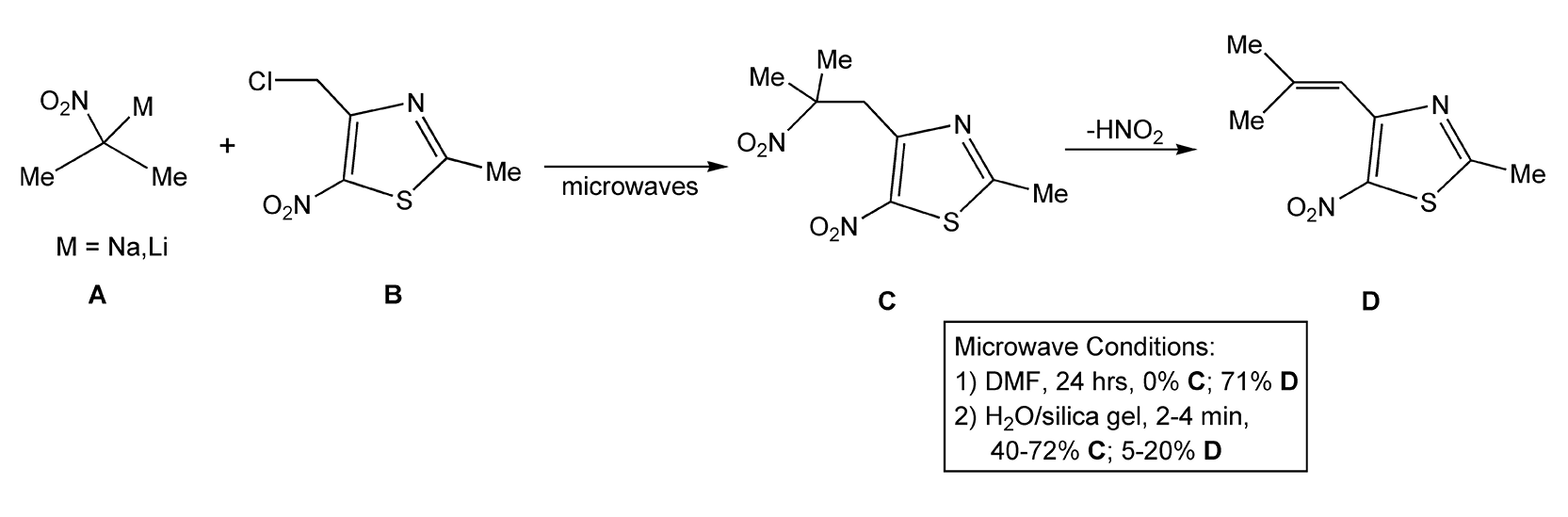
Scheme 75
Heterocyclic alkylation reactions also benefit from microwave irradiation. Saccharin can easily be alkylated with any alkyl halide under microwaves in only ten minutes (Scheme 76).445 The saccharin is first treated with base to form the sodium salt, which is then adsorbed on silica gel. This reaction is solvent-free and gives a 91% yield. Thiols can also be alkylated in near quantitative yield with alkyl halides via potassium carbonate on alumina (Scheme 77).450
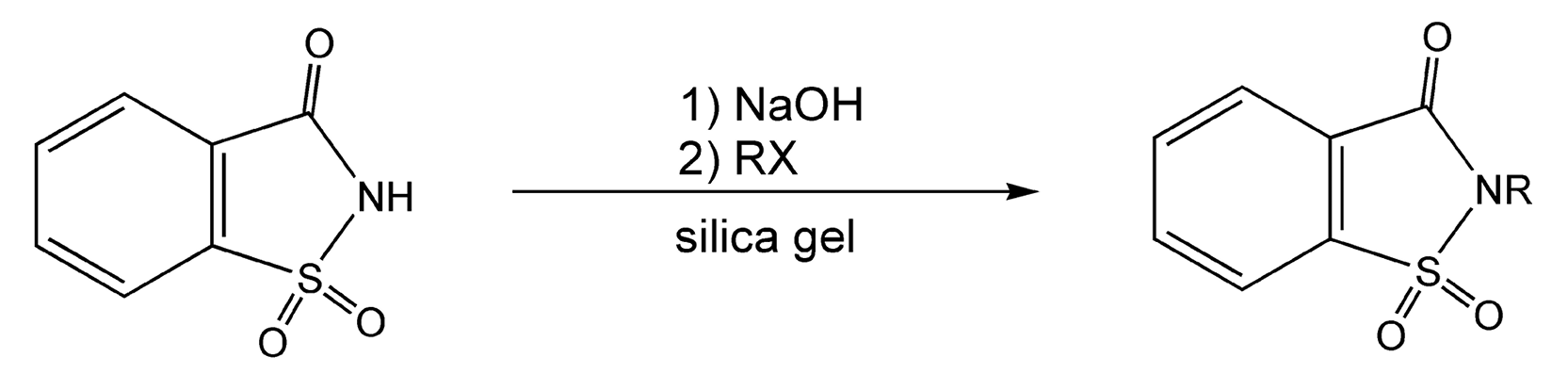
Scheme 76
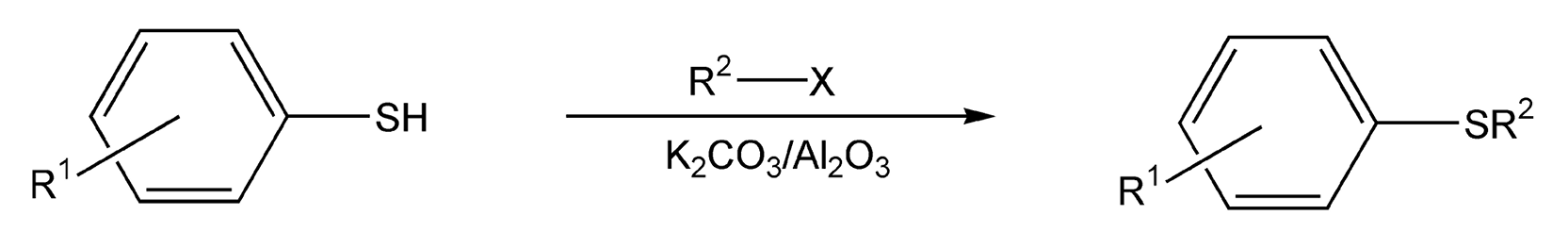
Scheme 77
Oxygen-alkylation of phenolic compounds is a versatile approach to aryl ethers. These compounds are the basis of many pharmaceutical templates that are used in drug discovery. With conductive heating, these reactions can take anywhere from one to seven days for completion. Microwave-enhanced transformations of a polymer-bound base (PTBD) with phenols occur in less than 30 minutes (Scheme 78).354
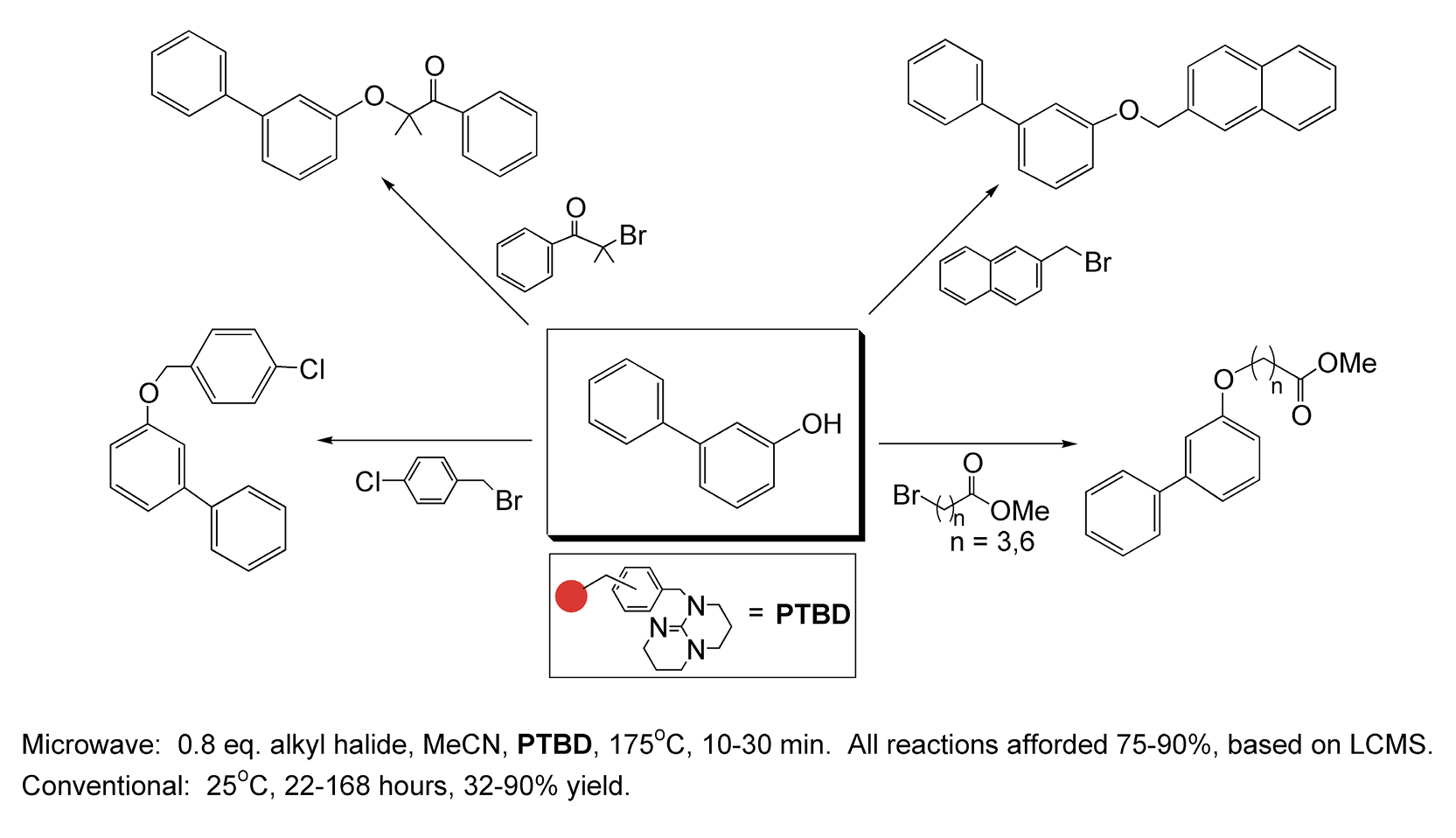
Scheme 78
The Williamson etherification reaction is another O-alkylation of alcohols, both alkyl and aromatic. Synthetically, it is a simple method to both symmetrical and asymmetrical ethers, but it can also be used in the protection of alcohols. Normally, with thermal heat, the reaction of alcohol with a primary alkyl halide and a base catalyst can take up to twelve hours before completion. With microwave irradiation, etherification of p-cresol is accomplished in three minutes (Scheme 79) and of sesamol in four minutes (Scheme 80).4,10,11,223
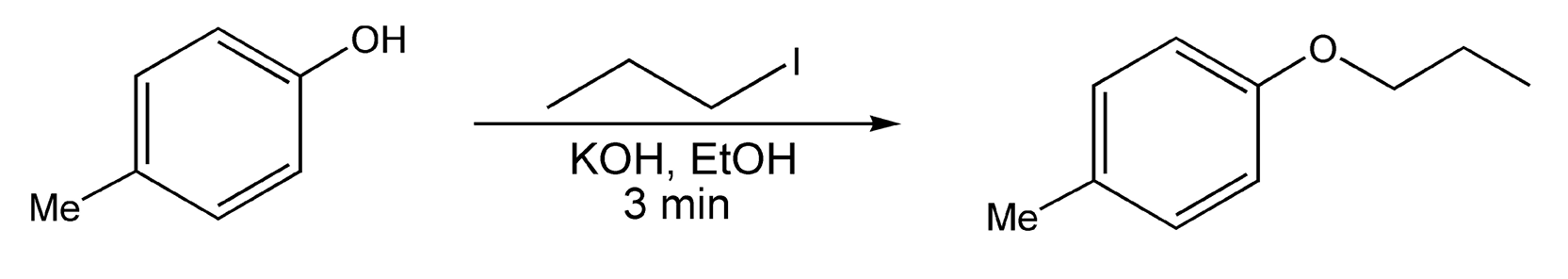
Scheme 79
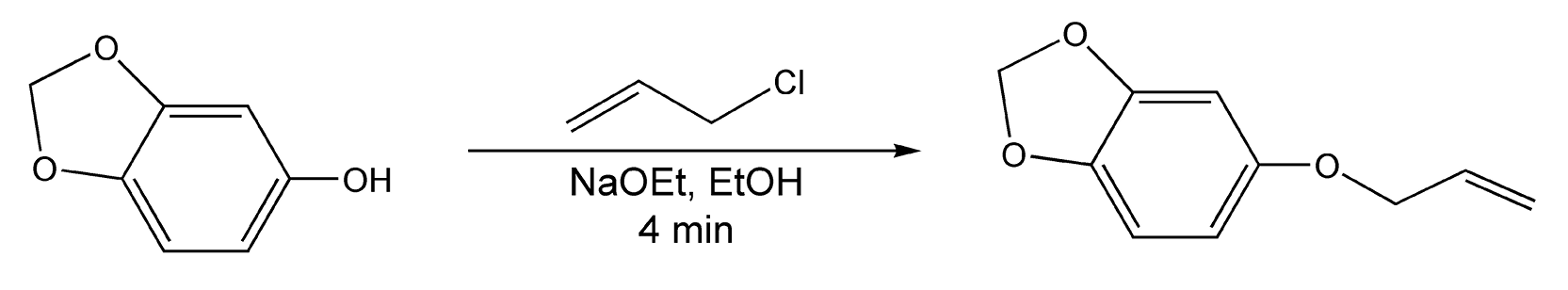
Scheme 80
Esters are very important organic molecules in both the chemical and pharmaceutical industry. Esterification of carboxylic acids, alkylation of carboxylate anions, and transesterifications are the three types of methods for ester synthesis. Fisher esterification reactions are direct transformations of carboxylic acids in a sulfuric acid/alcohol mixture. With conventional heating, these conditions are harsh and can take anywhere from two hours to two days. Loupy et al. using microwave irradiation and p-toluenesulfonic acid (PTSA), provided esters in near quantitative yields in ten minutes or less (Scheme 81).507
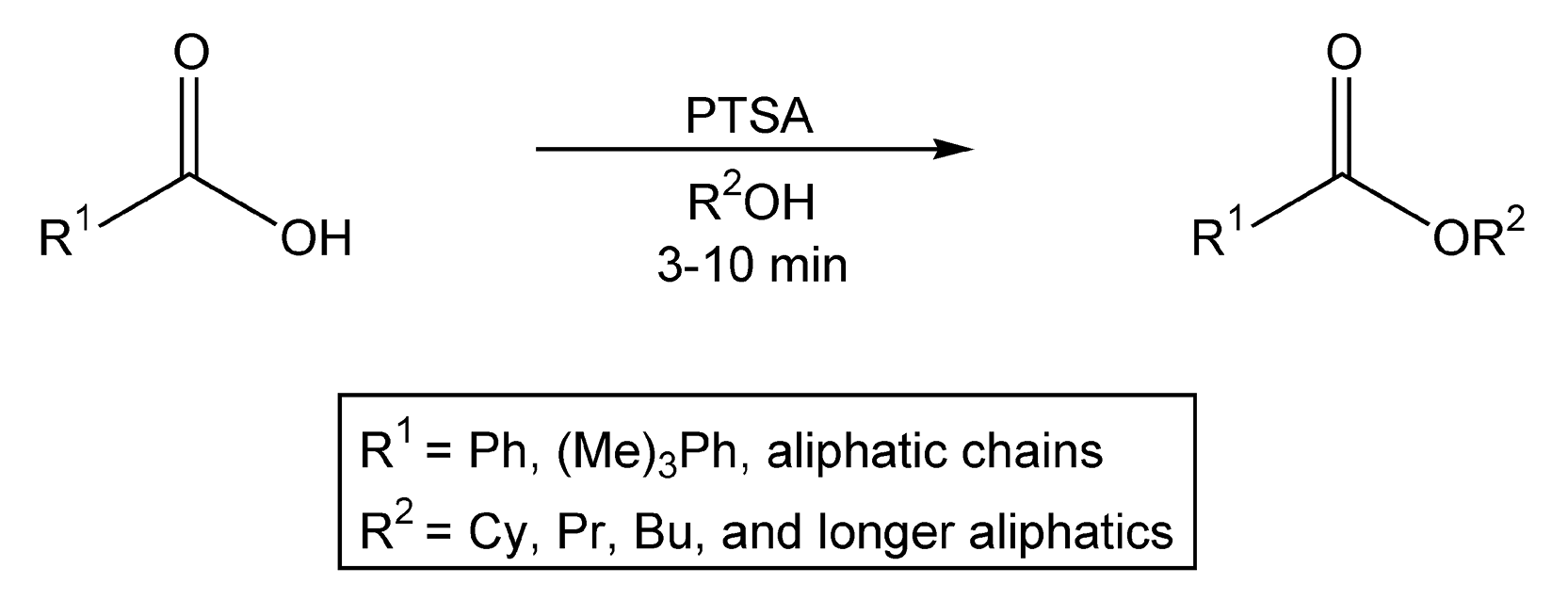
Scheme 81
Alkylation of carboxylate anions is another routine transformation to esters. Once again, Loupy and colleagues have extensively examined this area of esterification.505-507 Both potassium acetate (R1 = Me) and potassium benzoate (R1 = Ph), first generated in situ with either potassium hydroxide or potassium carbonate, were mixed with different alkyl halides. Under thermal conditions, esters are achieved in five hours; however, microwave-driven substitutions proceeded in 5-15 minutes on alumina (Scheme 82). Loupy and co-workers have also investigated microwave irradiation in transesterifications reactions.507 These reactions can be catalyzed by either an acid or a base, with PTSA and K2CO3, respectively, providing the most quantitative results. As shown in Scheme 83, the methoxy group of methyl benzoate is replaced by an octoxy group, which yields octyl benzoate and methanol. This reaction is successfully completed in two minutes with microwave heating.
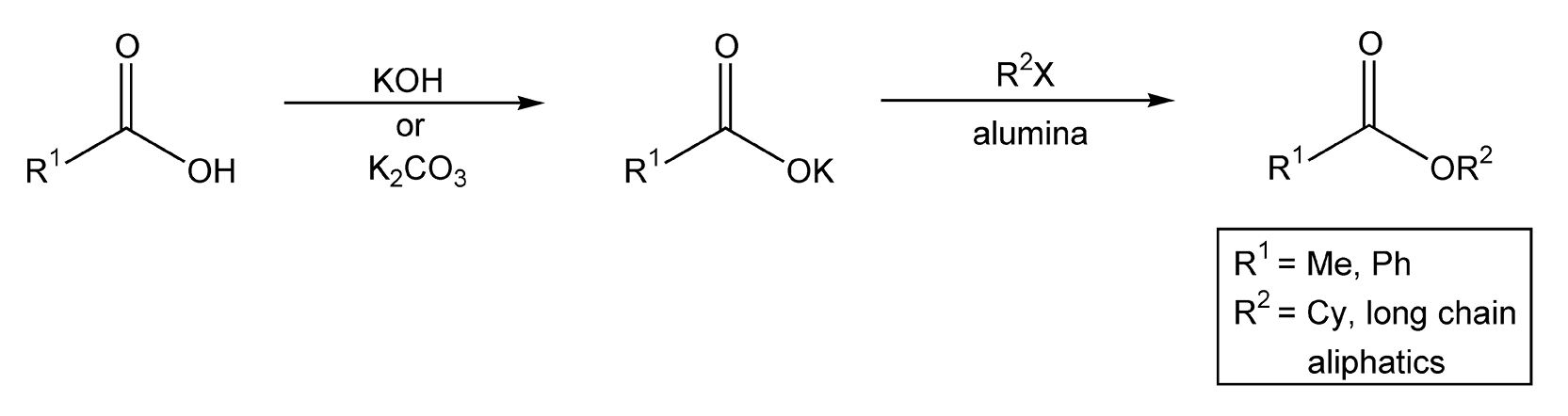
Scheme 82
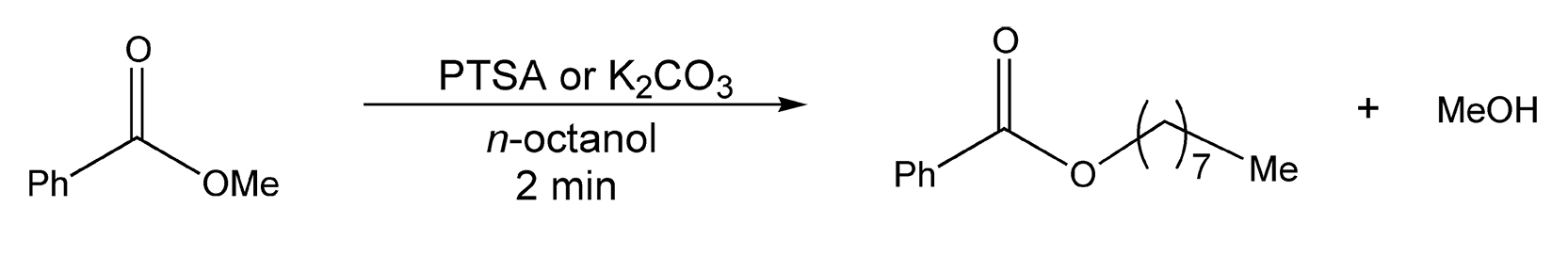
Scheme 83
The Finkelstein halogen exchange reaction is another nucleophilic substitution reaction. Alkyl iodides can be prepared easily from alkyl chlorides or bromides. This reaction is successful because, unlike sodium iodide, both sodium chloride and sodium bromide are not soluble in acetone or MEK. When an alkyl chloride or bromide is treated with sodium iodide, sodium chloride precipitates out of the solution, and formation of the alkyl iodide is favored. These reactions can take anywhere from 30 minutes to 80 hours for completion with conductive heating. Microwave heating yields alkyl iodides in ten minutes with excellent yields (Scheme 84).10,223
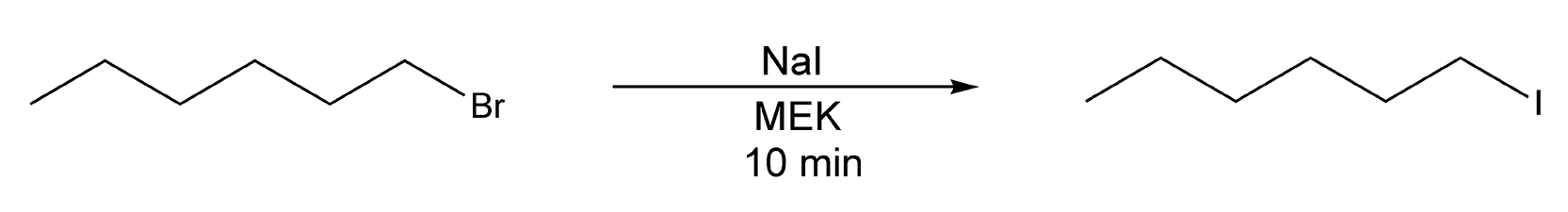
Scheme 84
Another example of nucleophilic substitution reaction is radiolabeling. It is always a challenge to synthesize radiopharmaceuticals that are labeled with short-lived radionuclei. These reactions typically require long reaction times, and thus, have low radiochemical yields. The use of microwave irradiation provides shorter reaction times, and as a result, higher radiochemical yields.519,520 SNAr reactions, with 18F-fluoride anion (via cyclotron), were performed on nitrobenzenes (Scheme 85).519 Comparing conventional methods to that of microwave heating, the radiochemical yields, in most cases, more than doubled with the use of microwaves. Scheme 86 shows the synthesis of epi-[18F]-fluoromisonidazole, which has been used in suspected cases of myocardial infarction.520 Synthetically, the yields increased from 40% (conventional) to 65% overall with microwave irradiation. In addition, the entire route, including work-up, took less than 70 minutes with a 40% radiochemical yield.
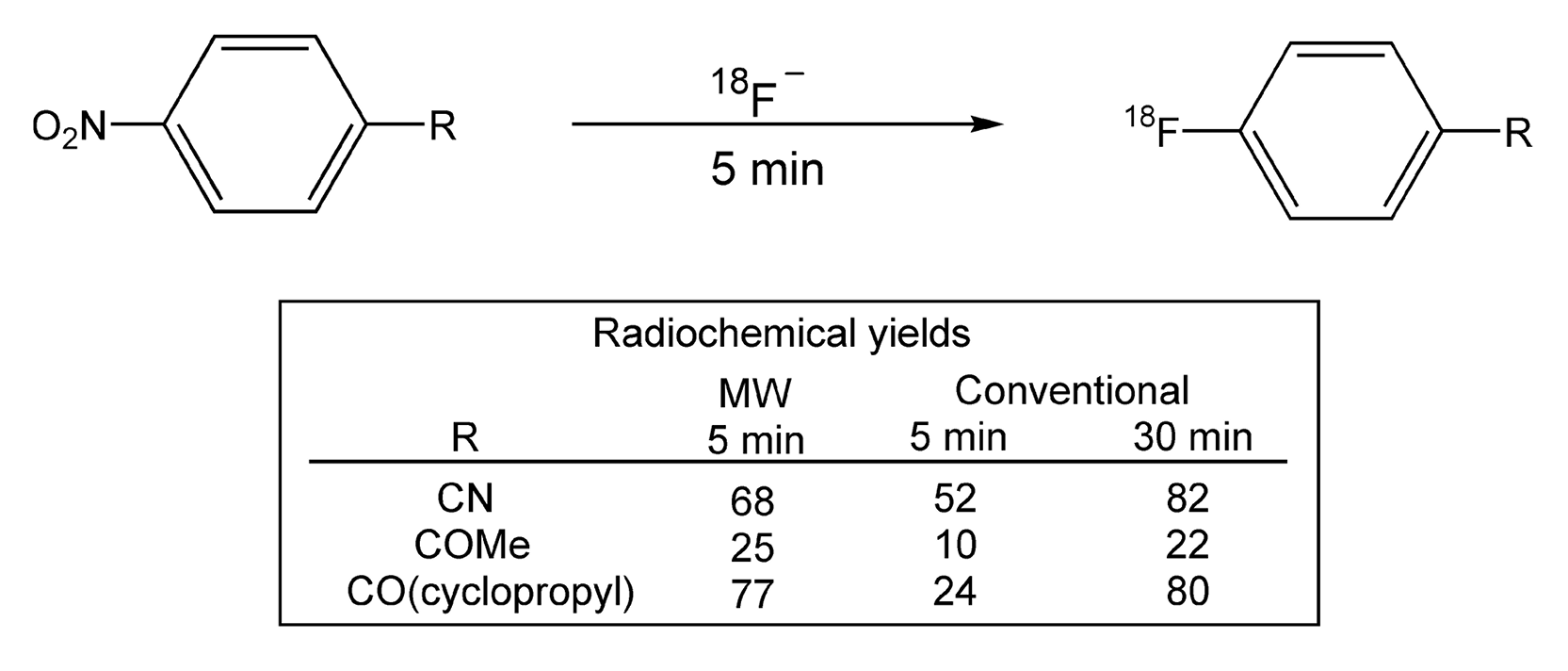
Scheme 85
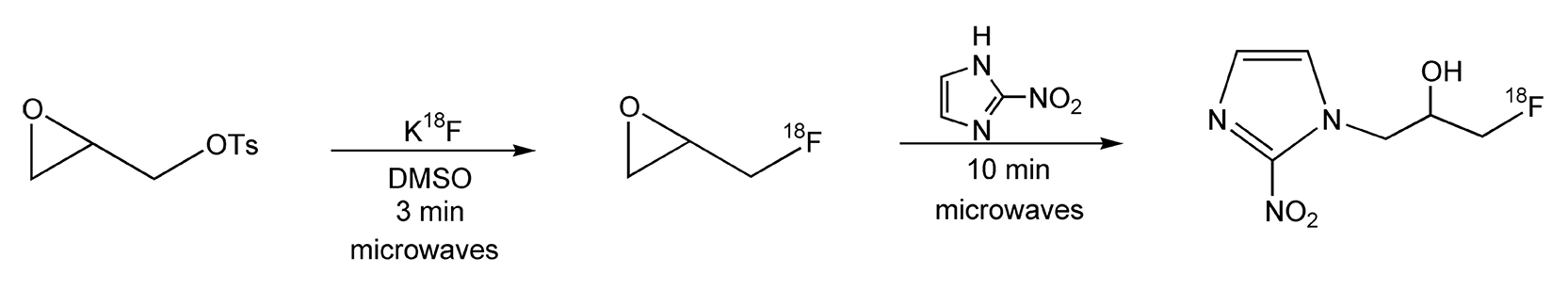
Scheme 86
Instruments
4. Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L.; Laberge, L.; Rousell, J. The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett. 1986, 27, pp. 279-82.
5. Jun, C.H.; Chung, J.H.; Lee, D.Y.; Loupy, A.; Chatti, S. “Solvent-free chelation-assisted intermolecular hydro-acylation: effect of microwave irradiation in the synthesis of ketone from aldehyde and 1-alkene by Rh(I) complex.” Tetrahedron Lett. 2001, 42, pp. 4803-05.
10. Majetich, G.; Hicks, R. “Applications of microwave-accelerated organic synthesis.” Radiat. Phys. Chem. 1995, 45, pp. 567-79.
11. Bose, A.K.; Manhas, M.S.; Ghosh, M.; Shah, M.; Raju, V.S.; Bari, S.S.; Newaz, S.N.; Banik, B.K.; Chaudhary, A.G.; Barakat, K.J. “Microwave-induced organic reaction enhancement chemistry. 2. Simplified techniques.” J. Org. Chem. 1991, 56, pp. 6968-70.
40. Scharn, D.; Wenschuh, H.; Reineke, U.; Schneider-Mergener, J.; Germeroth, L. “Spatially addressed synthesis of amino- and amino-oxy-substituted 1,3,5-triazine arrays on polymeric membranes.” J. Comb. Chem., 2000, 2, pp. 361-69.
41. Vanden Eynde, J.J.; Rutot, D. “Microwave-mediated derivatization of poly(styrene-co-allyl alcohol), a key step for the soluble polymer-assisted synthesis of heterocycles.” Tetrahedron 1999, 55, pp. 2687-94.
42. Yu, A.M.; Zhang, Z.P.; Yang, H.Z.; Zhang, C.X.; Liu, Z. “Wang resin bound addition reactions under microwave irradiation.” Synth. Commun. 1999, 29, 1595-99.
53. Gelo-Pujic, M.; Guibe-Jampel, E.; Loupy, A.; Trincone, A. “Enzymatic glycosidation in dry media under microwave irradiation.” J. Chem. Soc., Perkin Trans. 1 1997, pp. 1001-02.
54. Limousin, C.; Cleophaz, J.; Petit, A.; Loupy, A.; Lukacs, G. “Solvent-free synthesis of decyl D-glycopyranosides: under focused microwave irradiation.” J. Carbohydrate Chem. 1997, 16, pp. 327-42.
110. Kidwai, M., Bhushan, K.R.; Sapra, P.; Saxena, R.K.; Gupta, R. “Alumina-supported synthesis of antibacterial quinolines using microwaves.” Bioorg. Med. Chem. 2000, 8, pp. 69-72.
148. Michaud, D.; Abdallah-El Ayoubi, S.; Dozias, M.J.; Toupet, L.; Texier-Boullet, F.; Hamelin, J. “New route to functionalized cyclohexenes from nitromethane and electrophilic alkenes without solvent under focused microwave irradiation.” J. Chem. Soc., Chem. Commun. 1997, pp. 1613-14.
158. Gianotti, M.; Martelli, G.; Spunta, G.; Campana, E.; Panunzio, M.; Mendozza, M. “Solvent-free microwave-assisted organic reactions, preparation of β-keto-esters.” Synth. Commun. 2000, 30, pp. 1725-30.
172. Mojtahedi, M.M.; Saidi, M.R.; Bolourtchian, M. “Microwave assisted aminolysis of epoxides under solvent-free conditions catalyzed by montmorillonite clay.” J. Chem. Res. (S) 1999, pp. 128-29.
179. Nagy, G.; Filip, S.V.; Surducan, E.; Surducan, V. “Solvent-free synthesis of substituted phenoxyacetic acids under microwave irradiation.” Synth. Commun. 1997, 27, pp. 3729-36.
192. Olofsson, K.; Kim, S.Y.; Larhed, M.; Curran, D.P.; Hallberg, A. “High-speed, highly fluorous organic reactions.” J. Org. Chem. 1999, 64, pp. 4539-41.
379. Kidwai, M.; Sapra, P.; Dave, B. “A facile method for nucleophilic aromatic substitution of cyclic amine.” Synth. Commun. 2000, 30, pp. 4479-88.
380. Salmoria, G.V.; Dall’Oglio, E.L.; Zucco, C. “Aromatic nucleophilic substitutions under microwave irradiation.” Tetrahedron Lett. 1998, 39, pp. 2471-74.
381. Abramovitch, R.A.; Abramovitch, D.A.; Iyanar, K.; Tamareselvy, K. “Application of microwave energy to organic synthesis: improved technology.” Tetrahedron Lett. 1991, 32, pp. 5251-54.
382. Caddick, S. “Microwave assisted organic reactions.” Tetrahedron 1995, 51, pp. 10403-432.
383. Heber, D.; Stoyanov, E.V. “A microwave assisted nucleophilic substitution of 4-hydroxy-6-methyl-2(1H)-pyridones.” Synlett. 1999, 11, pp. 1747-48.
384. Yadav, J.S.; Reddy, B.V.S. “CsF-Al2O3 mediated rapid condensation of phenols with aryl halides: comparative study of conventional heating vs. microwave irradiation.” New J. Chem. 2000, 24, pp. 489-91.
385. Jiang, Y.L.; Pang, J.; Yuan, Y.C. “A novel preparation of o-ethoxyphenol from o-chlorophenol in the presence of phase transfer catalysts under microwave irradiation.” Chin. Chem. Lett. 1994, 5, pp. 29-30.
386. Sagar, A.D.; Patil, D.S.; Bandgar, B.P. “Microwave assisted synthesis of triaryl cyanurates.” Synth. Commun. 2000, 30, pp. 1719-23.
387. Bansal, V.; Kanodia, S.; Thapliyal, P.C.; Khanna, R.N. “Microwave induced selective bromination of 1,4-quinones and coumarins.” Synth. Commun. 1996, 26, pp. 887-92.
388. Chakrabarty, M.; Basak, R.; Ghosh, N. “Microwave-assisted Michael reactions of 3-(2’-nitrovinyl) indole with indoles on TLC-grade silica gel. A new, facile synthesis of 2,2-bis(3’-indolyl)nitroethanes.” Tetrahedron Lett. 2001, 42, pp. 3913-15.
389. de la Hoz, A.; Diaz-Ortiz, A.; Gomez, M.V.; Mayoral, J.A.; Moreno, A.; Sanchez-Migallon, A.M.; Vazquez, E. “Preparation of α- and β-substituted alanine derivatives by α-amidoalkylation or Michael addition reactions under heterogeneous catalysis assisted by microwave irradiation.” Tetrahedron 2001, 57, pp. 5421-28.
390. Ranu, B.C.; Guchhait, S.K.; Ghosh, K.; Patra, A. “Construction of bicyclo[2.2.2]octanone systems by microwave-assisted solid phase Michael addition followed by Al2O3-mediated intramolecular aldolisation. An eco-friendly approach.” Green Chem. 2000, 2, pp. 5-6.
391. Kayama, H.; Sawaguchi, M.; Nagata, C. “Acceleration of Michael addition reaction by microwave irradiation in the presence of metal acetylacetonate catalysts.” J. Chem. Soc. Japan, Chem. Indus. Chem. 1999, pp. 145-48.
392. Baruah, B.; Boruah, A.; Prajapati, D.; Sandhu, J.S. “BiCl3 or CdI2 catalyzed Michael additions of 1,3-dicarbonyl compounds under microwave irradiations.” Tetrahedron Lett. 1997, 38, pp. 1449-50.
393. Ibrahim-Ouali, M.; Sinibaldi, M.E.; Troin, Y.; Gardette, D.; Gramain, J.C. “Synthesis of β-enaminoesters and lactams by Michael addition of N-benzylaniline to new allenic esters and lactams.” Synth. Commun. 1997, 27, pp. 1827-48.
394. Ranu, B.C.; Saha, M.; Bhar, S. “Microwave-assisted Michael addition of cycloalkenones and substituted enones on the surface of alumina in dry media.” Synth. Commun. 1997, 27, pp. 621-26.
395. Soriente, A.; Spinella, A.; De Rosa, M.; Giordano, M.; Scettri, A. “Solvent-free reaction under microwave irradiation: A new procedure for Eu(III)-catalyzed Michael addition of 1,3-dicarbonyl compounds.” Tetrahedron Lett. 1997, 38, pp. 289-90.
396. Boruah, A.; Baruah, M.; Prajapati, D.; Sandhu, J.S. “Cerium catalyzed Michael addition of 1,3-dicarbonyl compounds under microwave irradiation.” Synth. Commun. 1998, 28, pp. 653-58.
397. Michaud, D.; Texier-Boullet, F.; Hamelin, J. “Michael monoaddition of nitromethane on gem-diactivated alkenes in dry media coupled with microwave irradiation.” Tetrahedron Lett. 1997, 38, pp. 7563-64.
398. Boruah, A.; Baruah, B.; Prajapati, D.; Sandhu, J.S. “Michael reaction in the solid state under microwave irradiations.” Chem. Lett. 1996, pp. 965-66.
399. Sviridova, L.A.; Golubeva, G.A. “New method for the direct cyanoethylation of pyrazole derivatives.” Chem. Heterocycl. Compd. 1999, 35, p. 245.
400. Moghaddam, F.M.; Mohammadi, M.; Hosseinnia, A. “Water promoted Michael addition of secondary amines to α,β-unsaturated carbonyl compounds under microwave irradiation.” Synth. Commun. 2000, 30, pp. 643-50.
401. Romanova, N.N.; Gravis, A.G.; Leshcheva, I.F.; Bundel, Y.G. “1,2-Asymmetric induction in nucleophilic Michael addition reactions of amines under microwave irradiation.” Mendeleev Commun. 1998, pp. 147-48.
402. Romanova, N.N.; Gravis, A.G.; Shaidullina, G.M.; Leshcheva, L.F.; Bundel, Y.G. “The application of microwave irradiation to the Michael synthesis of esters of amino acids.” Mendeleev Commun. 1997, pp. 235-36.
403. Steinreiber, A.; Stadler, A.; Mayer, S.F.; Faber, K.; Kappe, C.O. “High-speed microwave-promoted Mitsunobu inversions. Application toward the deracemization of sulcatol.” Tetrahedron Lett. 2001, 42, pp. 6283-86.
404. Jun, C.H.; Lee, H.; Hong, J.B. “Chelation-assisted intermolecular hydroacylation: direct synthesis of ketone from aldehyde and 1-alkene.” J. Org. Chem. 1997, 62, pp. 1200-01.
405. Borah, H.N.; Boruah, R.C.; Sandhu, J.S. “Microwave-induced one-pot synthesis of N-(carboxyalkyl)-maleimides and –phthalimides.” J. Chem. Res. (S) 1998, pp. 272-73.
406. Chandrasekhar, S.; Takhi, M.; Uma, G. “Solvent free N-alkyl and N-arylimide preparation from anhydrides catalyzed by TaCl5-silica gel.” Tetrahedron Lett. 1997, 38, pp. 8089-92.
407. Seijas, J.A.; Vázquez-Tato, M.P.; Martínez, M.M.; Núñez-Corredoira, G. “Direct synthesis of imides from dicarboxylic acids using microwaves.” J. Chem. Res. (S) 1999, pp. 420-21.
408. Bose, A.K.; Jayaraman, M.; Okawa, A.; Bari, S.S. “Microwave-assisted rapid synthesis of amino-lactams.” Tetrahedron Lett. 1996, 37, pp. 6989-92.
409. Vidal, T.; Petit, A.; Loupy, A.; Gedye, R.N. “Re-examination of microwave-induced synthesis of phthalimides.” Tetrahedron 2000, 56, pp. 5473-78.
410. Khajavi, M.S.; Nikpour, F.; Hajihadi, M. “Microwave irradiation promoted reactions of anhydrides with isocyanates. Preparation of N-substituted phthalimides.” J. Chem. Res. (S) 1996, pp. 96-97.
411. Kidwai, M.; Kumar, R. “Microwave-assisted synthesis of novel 1,3,4-thiadiazolyl-substituted-1,2,4-tetrazines, pyridazinones, 1,2,4-triazoles, 4-thiazolidinones, oxazoles, and thiazoles.” Gazz. Chim. Ital. 1997, 127, pp. 263-68.
412. Mojtahedi, M.M.; Saidi, M.R.; Bolourtchian, M. “A novel method for the synthesis of disubstituted ureas and thioureas under microwave irradiaton.” J. Chem. Res. (S) 1999, pp. 710-11.
413. Marquez, H.; Plutin, A.M.; Rodriguez, Y.; Perez, E.; Loupy, A. “Efficient synthesis of 1-(4’-methylbenzoyl)-3,3-diethylthiourea under microwave irradiation using potassium fluoride on alumina.” Synth. Commun. 2000, 30, pp. 1067-74.
414. Kidwai, M.; Goel, Y.; Kumar, R. “Microwave-assisted synthesis and antifungal activity of 1,2,4-triazine, 1,2,4-triazole, tetrazole, and pyrazole derivatives.” Indian J. Chem. 1998, 37B, pp. 174-79.
415. Williams, L. “Thin layer chromatography as a tool for reaction optimisation in microwave assisted synthesis.” J. Chem. Soc., Chem. Commun. 2000, pp. 435-36.
416. Marquez, H.; Perez, E.R.; Plutin, A.M.; Morales, M.; Loupy, A. “Synthesis of 1-benzoyl-3-alkylthioureas by transamidation under microwave in dry media.” Tetrahedron Lett. 2000, 41, pp. 1753-56.
417. Gadhwal, S.; Dutta, M.P.; Boruah, A.; Prajapati, D.; Sandhu, J.S. “Zeolite-HY: a selective and efficient catalyst for the synthesis of amides under microwave irradiations.” Indian J. Chem. 1998, 37B, pp. 725-27.
418. Baldwin, B.W.; Hirose, T.; Wang, Z.H. “Improved microwave oven synthesis of amides and imides promoted by imidazole; convenient transport agent preparation.” J. Chem. Soc., Chem. Commun. 1996, pp. 2669-70.
419. Dayal, B.; Rapole, K.R.; Patel, C.; Pramanik, B.N. “Microwave-induced rapid synthesis of sarcosine conjugated bile acids.” Bioorg. Med. Chem. Lett. 1995, 5, pp. 1301-06.
420. Linares, R.M.; Ayala, J.H.; Afonso, A.M.; Gonzalez, V. “Quantitative analysis of biogenic amines by high-performance thin-layer chromatography utilizing a fibre optic fluorescence detector.” Anal. Chem. 1998, 31, pp. 475-89.
421. Kidwai, M.; Kumar, R.; Srivastava, A.; Gupta, H.P. “Microwave-assisted synthesis of novel 1,3,4-thiadi-azolyl-substituted-1,2,4-triazines as potential antitubercular agents.” Bioorg. Chem. 1998, 26, pp. 289-94.
422. Iranpoor, N.; Zeynizadeh, B. “Microwave-promoted trifluoroacetylation of amines with TiO(CF3CO2)2.” J. Chem. Res. (S) 1999, pp. 124-25.
423. Kalita, D.J.; Borah, R.; Sarma. J.C. “A selective catalytic method of enol-acetylation under microwave irradiation.” J. Chem. Res. (S) 1999, 404-05.
424. Deka, N.; Mariotte, A.M.; Boumendjel, A. “Microwave-mediated solvent-free acetylation of deactivated and hindered phenols.” Green Chem. 2001, 3, pp. 263-64.
425. Oussaid, A.; Pentek, E.; Loupy, A. “Selective alkylations of 2-naphthol using solvent-free conditions under microwave irradiation.” New J. Chem. 1997, 21, pp. 1339-45.
426. Vanelle, P.; Gellis, A.; Kaafarani, M.; Maldonado, J.; Crozet, M.P. “Fast electron transfer C-alkylation of 2-nitropropane anion under microwave irradiation.” Tetrahedron Lett. 1999, 40, pp. 4343-46.
427. Bansal, V.; Singh, P.K.; Khanna, R.N. “New synthesis of 1,3-diarylpropane-1,3-diones.” Indian J. Chem. 1996, 35B, pp. 586-87.
428. Deng, R.; Wang, Y.; Jiang, Y. “Solid-liquid phase transfer catalytic synthesis. X. The rapid alkylation of ethyl acetoacetate under microwave irradiation.” Synth. Commun. 1994, 24, pp. 111-15.
429. Abramovitch, R.A.; Shi, Q.; Bogdal, D. “Microwave-assisted alkylations of activated methylene groups.” Synth. Commun. 1995, 25, pp. 1-8.
430. Kumar, H.M.S.; Reddy, B.V.S.; Reddy, E.J.; Yadav, J.S. “Microwave-assisted eco-friendly synthesis of 2-alkylated hydroquinones in dry media.” Green Chem. 1999, 1, pp. 141-42.
431. Abdallah-El Ayoubi, S.; Toupet, L.; Texier-Boullet, F.; Hamelin, J. “New route to functionalized cyclohexenes in solvent-free conditions from enamino ketones and oxo alkenes.” Synthesis 1999, 7, pp. 1112-16.
432. Ruault, P.; Pilard, J.F.; Touaux, B.; Texier-Boullet, F.; Hamelin, J. “Rapid generation of amines by microwave irradiation of ureas dispersed on clay.” Synlett. 1994, 11, pp. 935-36.
433. Mojtahedi, M.M.; Sharifi, A.; Mohsenzadeh, F.; Saidi, M.R. “Microwave-assisted aminomethylation of electron-rich compounds under solvent-free condition.” Synth. Commun. 2000, 30, pp. 69-72.
434. Villemin, D.; Sauvaget, F. “Dry synthesis under microwave irradiation: a rapid and efficient coupling of naphthols.” Synlett. 1994, 6, pp. 435-36.
435. Cado, F.; Di-Martino, J.L.; Jacquault, P.; Bazureau, J.P.; Hamelin, J. “Amidine-enediamine tautomerism: addition of isocyanates to 2-substituted 1H-perimidines. Some syntheses under microwave irradiation.” Bull. Soc. Chim. Fr. 1996, 133, pp. 587-95.
436. Carrillo, J.R.; Diaz-Ortiz, A.; de la Hoz, A.; Gomez-Escalonilla, M.J.; Moreno, A.; Prieto, P. “The effect of focused microwaves on the reaction of ethyl N-trichloroethylidenecarbamate with pyrazole derivatives.” Tetrahedron 1999, 55, pp. 9623-30.
437. Kundu, M.K.; Mukherjee, S.B.; Balu, N.; Padmakumar, R. “Microwave mediated extensive rate enhancement of Baylis-Hillman reaction.” Synlett. 1994, 6, p. 444.
438. Garrigues, B.; Laurent, R.; Laporte, C.; Laporterie, A.; Dubac, J. “Microwave-assisted carbonyl Diels-Alder and carbonyl-ene reactions supported on graphite.” Liebigs Ann. 1996, pp. 743-44.
439. Almena, I.; Diaz-Ortiz, A.; Diez-Barra, E.; de la Hoz, A.; Loupy, A. “Solvent-free benzylations of 2-pyridone. Regiospecific N- or C-alkylation.” Chem. Lett. 1996, pp. 333-34.
440. Torchy, S.; Barbry, D. “N-alkylation of amines under microwave irradiation: modified Eschweiler-Clarke reaction.” J. Chem. Res. (S) 2001, pp. 292-93.
441. Fan, X.J.; You, J.M.; Jiao, T.Q.; Tan, G.Z.; Yu, X.D. “Rapid N-alkylation of carbazole, phenothiazine, and acridone under microwave irradiation.” Org. Prep. Proc. Intl. 2000, 32, pp. 284-87.
442. Bogdal, D.; Pielichowski, J.; Jaskot, K. “Remarkable fast N-alkylation of azaheterocycles under microwave irradition in dry media.” Heterocycles 1997, 45, pp. 715-22.
443. Bogdal, D.; Pielichowski, J.; Jaskot, K. “New synthesis method of N-alkylation of carbazole under microwave irradition in dry media.” Synth. Commun. 1997, 27, pp. 1553-60.
444. Bogdal, D.; Pielichowski, J.; Boron, A. “Remarkable fast microwave-assisted N-alkylation of phthalimide in dry media.” Synlett. 1996, 37, pp. 873-74.
445. Ding, J.; Gu, H.; Wen, J.; Lin, C. “Dry reaction under microwave: N-alkylation of saccharin on silica gel.” Synth. Commun. 1994, 24, pp. 301-03.
446. Gupta, R.; Paul, S.; Gupta, A.K.; Kachroo, P.L.; Dandia, A. “Opening of oxirane ring with N-nucleophiles under microwave irradiation.” Indian J. Chem. 1997, 36B, pp. 281-83.
447. Sabitha, G.; Reddy, B.V.S.; Abraham, S.; Yadav, J.S. “Microwave promoted synthesis of aminoalcohols in dry media.” Green Chem. 1999, 1, pp. 251-52.
448. Lindström, U.M.; Olofsson, B.; Somfai, P. “Microwave-assisted aminolysis of vinylepoxides.” Tetrahedron Lett. 1999, 40, pp. 9273-76.
449. Yadav, J.S.; Reddy, B.V.S. “Microwave-assisted efficient synthesis of N-arylamines in dry media.” Green Chem. 2000, 2, pp. 115-16.
450. Jaisinghani, H.G.; Khadilkar, B.M. “Microwave-assisted, highly efficient solid state N- and S-alkylation.” Synth. Commun. 1999, 29, pp. 3693-98.
451. Adamczyk, M.; Rege, S. “Microwave assisted sulfopropylation of N-heterocycles using 1,3-propane sultone.” Tetrahedron Lett. 1998, 39, pp. 9587-88.
452. Wang, C.D.; Lu, J.; Shi, X.Z.; Feng, Y.H. “Synthesis of thiosemicarbazones under microwave irradiation.” Synth. Commun. 1999, 29, pp. 3057-61.
453. Molina, P.; Fresneda, P.M.; Delgado, S. “Iminophosphorane-mediated synthesis of the alkaloid cryptotackieine.” Synthesis 1999, 2, pp. 326-29.
454. Forfar, I.; Cabildo, P.; Claramunt, R.M.; Elguero, J. “Synthesis of 3-(1-adamantyl)pyrazole and 3,5-di(1-adamantyl)pyrazole in a microwave oven.” Chem. Lett. 1994, pp. 2079-80.
455. Almena, I.; Diez-Barra, E.; de la Hoz, A.; Riuz, J.; Sanchez-Migallon, A.; Elguero, J. “Alkylation and arylation of pyrazoles under solvent-free conditions: conventional heating versus microwave irradiation.” J. Heterocycl. Chem. 1998, 35, pp. 1263-68.
456. Abenhaim, D.; Diez-Barra, E.; de la Hoz, A.; Loupy, A.; Sanchez, M.A. “Selective alkylation of 1,2,4-triazole and benzotriazole in the absence of solvent.” Heterocycles 1994, 38, pp. 793-802.
457. Stankovicova, H.; Gasparova, R.; Lacova, M.; Chovancova, J. “Reaction of 4-oxochromene-3-carboxaldehydes with primary amides and benzotriazole or 1H-1,2,4-triazole.” Collect. Czech. Chem. Commun. 1997, 62, pp. 793-802.
458. Kornet, M.J. “Microwave synthesis and anticonvulsant activity of new 3-benzyl-1,2,3-benzotriazin-4(3H)ones.” J. Heterocycl. Chem. 1997, 34, pp. 1391-93.
459. Kornet, M.J.; Shackleford, G. “Microwave synthesis and anticonvulsant activity of new 2-benzyl-1(2H)-phthalazinones.” J. Heterocycl. Chem. 1999, 36, pp. 1095-96.
460. Jiang, Y.L.; Hu, Y.Q.; Feng, S.Q.; Wu, J.S.; Wu, Z.W.; Yuan, Y.C. “Facile N-alkylation of anilines with alcohols over Raney nickel under microwave irradiation.” Synth. Commun. 1996, 26, pp. 161-64.
461. Barbry, D.; Torchy, S. “Fast N-methylation of amines under microwave irradiation.” Synth. Commun. 1996, 26, pp. 3919-22.
462. Stone-Elander, S.A.; Elander, N.; Thorell, J.O.; Solas, G.; Svennebrink, J. “A single-mode microwave cavity for reducing radiolabeling reaction times, demonstrated by alkylation with (11C)alkyl halides.” J. Labelled Compd. Radiopharm. 1994, 34, pp. 949-60.
463. Kidwai, M.; Kumar, P.; Goel, Y.; Kumar, K. “Microwave assisted synthesis of 5-methyl-1,2,4-thiadiazol-2-yl/thiotetrazol-1-yl substituted pyrazoles, 2-azetidinones, 4-thiazolidinones, benzopyran-2-ones, and 1,3,4-oxadiazoles.” Indian J. Chem. 1997, 36B, pp. 175-79.
464. Sharifi, A.; Mirzaei, M.; Nairni-Jamal, M.R. “Solvent-free aminoalkylation of phenols and indoles assisted by microwave irradiation.” Monatsh. Chem. 2001, 132, pp. 875-80.
465. Kidwai, M.; Misra, P.; Kumar, R.; Saxena, R.K.; Gupta, R.; Bradoo, S. “Microwave assisted synthesis and antibacterial activity of new quinolone derivatives.” Monatsh. Chem. 1998, 129, pp. 961-66.
466. Kidwai, M.; Kohli, S. “Environmentally co-friendly thiolation of 1,4-naphthoquinone.” Indian J. Chem. 1998, 37B, pp. 1294-95.
467. Kidwai, M.; Bhushan, K.R.; Misra, P. “A rapid and cheap synthesis of cephalosporins.” Chem. Lett. 1999, pp. 487-88.
468. Kumar, P.; Gupta, K.C. “Microwave assisted synthesis of S-trityl and S-acylmercapto alkanols, nucleosides and their deprotection.” Chem. Lett. 1996, pp. 635-36.
469. Soukri, M.; Guillaumet, G.; Besson, T.; Aziane, D.; Aadil, M.; Essassi, E.M.; Akssira, M. “Synthesis of novel 5a,10,14b,15-tetraaza-benzo[a]indeno[1,2-c]anthracen-5-one and benzimidazo[1,2-c]quinazoline derivatives under microwave irradiation.” Tetrahedron Lett. 2000, 41, pp. 5857-60.
470. Vanden Eynde, J.J.; Mailleux, I. “Quaternary ammonium salt-assisted organic reactions in water: alkylation of phenols.” Synth. Commun. 2001, 31, pp. 1-7.
471. Lima, L.M.; Barreiro, E.J.; Fraga, C.A.M. “O-alkylation of bioactive phthalimide derivatives under microwave irradiation in dry media.” Synth. Commun. 2000, 30, pp. 3291-306.
472. Chatti, S.; Bortolussi, M.; Loupy, A. “Synthesis of diethers derived from dianhydrohexitols by phase transfer catalysis under microwave.” Tetrahedron Lett. 2000, 41, pp. 3367-70.
473. Mitra, A.K.; De, A.; Karchaudhuri, N. “Microwave enhanced synthesis of aromatic methyl ether.” Indian J. Chem. Sect. B 2000, 39, pp. 387-89.
474. Motorina, I.A.; Parly, F.; Grierson, D.S. “Selective O-allylation of amido alcohols on solid support.” Synlett. 1996, 4, pp. 389-91.
475. Khadilkar, B.M.; Bendale, P.M. “Microwave enhanced synthesis of epoxypropoxyphenols.” Synth. Commun. 1997, 27, pp. 2051-56.
476. Wang, J.X.; Zhang, Y.; Huang, D.; Hu, Y. “Solid-liquid phase-transfer catalytic synthesis of chiral glycerol sulfide ethers under microwave irradiation.” J. Chem. Res. (S) 1998, pp. 216-17.
477. Pchelka, B.; Plenkiewicz, J. “Microwave-promoted synthesis of 1-aryloxy-3-alkylamino-2-propanols.” Org. Prep. Proced. Int. 1998, 30, pp. 87-89.
478. Bagnell, L.; Cablewski, T.; Strauss, C.R. “A catalytic symmetrical etherification.” J. Chem. Soc., Chem. Commun. 1999, pp. 283-84.
479. Zadmard, R.; Aghapoor, K.; Bolourtchian, M.; Saidi, M.R. “Solid composite copper-copper chloride assisted alkylation of naphthols promoted by microwave irradiation.” Synth. Commun. 1998, 28, pp. 4495-99.
480. Wang, Z.Y.; Shi, H.J.; Shi, H.X.; Zhang, Z.Y. “The synthesis of aryloxycarboxylic acid under microwave, solid base as a support.” Chin. Chem. Lett. 1996, 7, pp. 527-30.
481. Elder, J.W.; Holtz, K.M. “Microwave microscale organic experiments.” J. Chem. Educ. 1996, 73, pp. A104-A105.
482. Khalafi-Nezhad, A.; Hashemi, A. “Efficient synthesis of sodium aryloxymethanesulfonates using microwave irradiation.” J. Chem. Res. (S) 1999, pp. 720-21.
483. Pang, J.; Xi, Z.; Cao, G.; Yuan, Y. “Phase transfer catalyzed synthesis of o-ethoxyphenol under microwave irradiation.” Synth. Commun. 1996, 26, pp. 3425-29.
484. Kidwai, M.; Kumar, P.; Kohli, S. “Microwave-induced selective alkoxylation of 1,4-naphthoquinones.” J. Chem. Res. (S) 1997, pp. 24-25.
485. Bansal, V.; Sharma, J.; Khanna, R.N. “Microwave-induced monohydroxymethylation and monoalkoxylation of 1,4-naphthoquinones.” J. Chem. Res. (S) 1998, pp. 720-21.
486. Wang, J.X.; Zhang, M.; Xing, Z.; Hu, Y. “Synthesis of aromatic ethers without organic solvent and inorganic carrier under microwave irradiation.” Synth. Commun. 1996, 26, pp. 301-05.
487. Bratulescu, G. “Organic synthesis in the absence of solvent and absorbent support via microwave activation. Application to the synthesis of azoxy ethers.” Rev. Roum. Chim. 1998, 43, pp. 1153-56.
488. Bogdal, D.; Pielichowski; J.; Boron, A. “New synthetic method of aromatic ethers under microwave irradiation in dry media.” Synth. Commun. 1998, 28, pp. 3029-39.
489. Wang, J.X.; Zhang, M.; Hu, Y. “Synthesis of 8-quinolinyl ethers under microwave irradiation.” Synth. Commun. 1998, 28, pp. 2407-13.
490. Reddy, Y.T.; Rao, M.K.; Rajitha, B. “Facile synthesis of desyl ethers under phase transfer catalytic and solvent free microwave conditions.” Indian J. Heterocycl. Chem. 2000, 101, pp. 73-74.
491. Majdoub, M.; Loupy, A.; Petit, A.; Roudesli, S. “Coupling focused microwaves and solvent-free phase transfer catalysis: application to the synthesis of new furanic diethers.” Tetrahedron 1996, 52, pp. 617-28.
492. Stadler, A.; Kappe, C.O. “The effect of microwave irradiation on carbodiimide-mediated esterifications on solid support.” Tetrahedron 2001, 57, pp. 3915-20.
493. Fan, X.J.; You, J.M.; Jiao, T.Q.; Tan, G.Z.; Yu, X.D. “Esterification by microwave irradiation on activated carbon.” Org. Prep. Proc. Intl. 2000, 32, pp. 287-90.
494. Kabza, K.G.; Chapados, B.R.; Gestwicki, J.E.; McGrath, J.L. “Microwave-induced esterification using heterogeneous acid catalyst in a low dielectric constant medium.” J. Org. Chem. 2000, 65, pp. 1210-14.
495. Mitra, A.K.; De, A.; Karchaudhuri, N. “Microwave enhanced esterification of α,β-unsaturated acids.” Indian J. Chem. Sect. B 2000, 39, pp. 311-12.
496. Lami, L.; Casal, B.; Cuadra, L.; Merino, J.; Alvarez, A.; Ruiz-Hitzky, E. “Synthesis of 2,4-D-ester herbicides - new routes using inorganic solid supports.” Green Chem. 1999, 1, pp. 199-204.
497. Chemat, F.; Poux, M.; Galema, S.A. “Esterification of stearic acid by isomeric forms of butanol in a microwave oven under homogeneous and heterogeneous reaction conditions.” J. Chem. Soc., Perkin Trans. 2 1997, pp. 2371-74.
498. Jiang, Y.L.; Yuan, Y.C. “The tribromolanthanoids (LnBr3) catalyzed reactions of benzyl alkyl ethers and carboxylic acids promoted by microwave irradiation.” Synth. Commun. 1994, 24, pp. 1045-48.
499. Kwon, P.S.; Kim, J.K.; Kwon, T.W.; Kim, Y.H.; Chung, S.K. “Microwave-irradiated acetylation and nitration of aromatic compounds.” Bull. Korean Chem. Soc. 1997, 18, pp. 1118-19.
500. Dasgupta, A.; Thompson, W.C.; Malik, S. “Use of microwave irradiation for rapid synthesis of perfluorooctanoyl derivatives of fatty alcohols, a new derivative for gas chromatograpy-mass spectrometric and fast atom bombardment mass spectrometric study.” J. Chromatogr. A 1994, 685, pp. 279-85.
501. Gelo-Pujic, M.; Guibe-Jampel, E.; Loupy, A.; Galema, S.A.; Mathe, D. “Lipase-catalyzed esterification of some α-D-glucopyranosides in dry media using focused microwave irradiation.” J. Chem. Soc., Perkin Trans. 1 1996, pp. 2777-80.
502. Herradon, B.; Morcuende, A.; Valverde, S. “Microwave accelerated organic transformations:

