Solvent and Solvent Free (Neat) Reactions in Microwave Synthesis
The two main types of conditions used for chemical reactions, those run in the presence of solvent and those run in a solventless environment, are equally important and both can benefit from microwave heating.
Microwave irradiation is not only applicable to standard solution-phase work, but to solid-phase and solvent-free systems as well. Many synthetic methods can be executed by at least one of these systems, though one may have to experiment in order to find the optimal conditions. This chapter discusses the different types of reaction conditions that can be used successfully with microwave irradiation and should be utilized in conjunction with Chapter 4 for synthetic applications. Note: The reader should assume that all reaction schemes shown in this chapter utilize microwave irradiation. In multi-step schemes, the use of microwaves is indicated by the word “microwaves” on the arrow.
Reactions in the presence of solvent
Solution-phase reactions performed in the presence of solvent can be either homogeneous or heterogeneous. Homogeneous reactions include your standard organic reactions in which all reagents are dissolved in the solvent. Microwave irradiation has been used extensively and successfully with homogeneous solution-phase reactions. Chapter 4 provides an in-depth review of the many homogeneous synthetic applications that have been enhanced with microwaves.
Heterogeneous reactions in solution involve insoluble solids that are used as reagents, catalysts, or supports. These include transition-metal and Lewis acid catalysts, non-dissolvable salts, and solid-phase resins (beads, lanterns, crowns, pins). These types of transformations are widely used and highly successful. One of the main disadvantages in traditional heterogeneous reactions are the long reaction times that are required for their completion, which is largely due to the insolubility. Use of microwave irradiation has been shown to drastically speed up these reactions, allowing for productive high throughput synthesis.
The following chapter on synthetic applications extensively covers heterogeneous reactions involving transition-metal catalysts, as well as those that utilize Lewis acids and other insoluble salts. It also provides a few examples of solid-phase reactions.188,204,609,631 The remainder of this section will provide a more detailed review on microwave-enhanced reactions that have been performed on a solid-phase resin.27-42
Combinatorial chemistry on solid-phase supports was first applied to peptide synthesis. It made sense to use peptides in the first microwave-assisted, solid-phase reaction. Traditional peptide hydrolysis requires high temperature 6M HCl for at least 24 hours. In 1988, Yu et al. performed a successful hydrolysis in seven minutes in a domestic microwave oven (Scheme 6).27 The same group, four years later, performed peptide coupling reactions in quantitative yields with microwave irradiation (Scheme 7).28
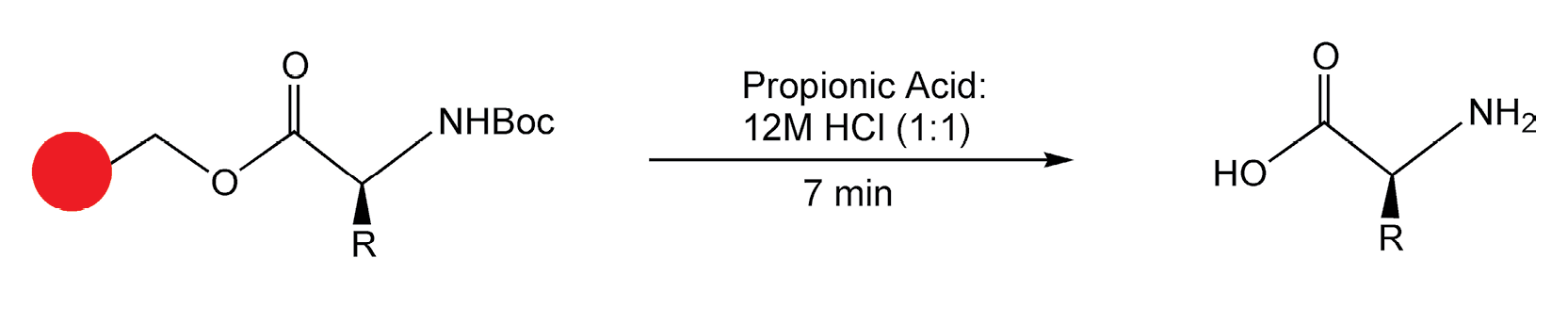
Scheme 6
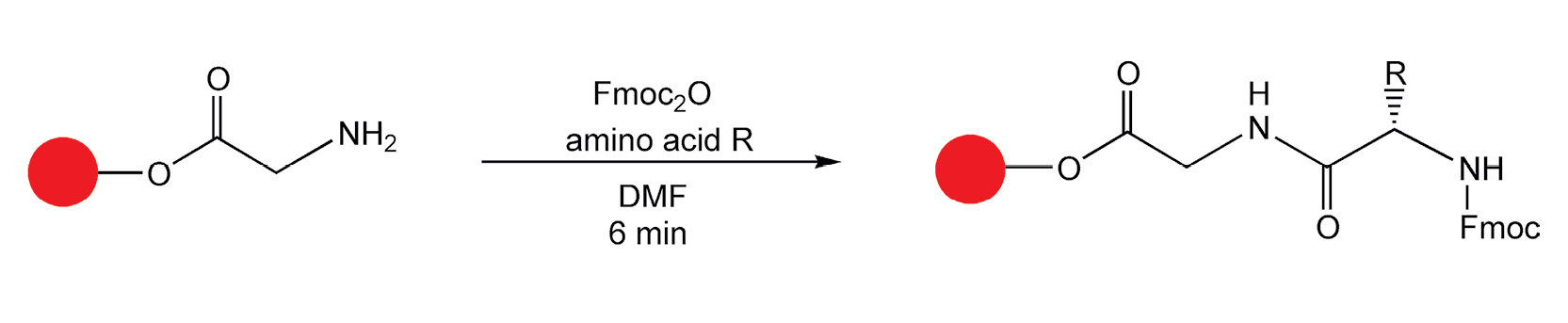
Scheme 7
Sigmatropic rearrangements are important pericyclic reactions that involve the formation of new carbon–carbon bonds. Sometimes, conventional methods require very long reaction times. Microwave irradiation has been used to facilitate these transformations, and this is outlined in the next chapter. Claisen rearrangements have been successfully performed on a solid phase resin coupled with microwave heating. Scheme 8 illustrates the rearrangement of resin bound O-allylic aryl ethers to ortho-allylic salicylic acid derivatives, where the allyl, hydroxyl, and carboxylic acid groups are adjacent to each other.38 These compounds are difficult to synthesize with traditional aromatic substitution reactions.

Scheme 8
Strohmeier and Kappe have performed an important example of microwave-assisted, solid-phase parallel synthesis.29 Enones, as well as their 1,3-dicarbonyl intermediates, are important building blocks for heterocyclic scaffolds that are used in pharmaceutical drug design. Conventional solid-phase methods require multiple steps and long high-temperature reaction times. In this microwave-enhanced, two-step procedure, acetoacetylation of a polystyrene Wang (PS-Wang) resin to a functionalized β-ketoester, followed by Knoevenagel condensation with an aldehyde, yields enones in less than one hour (Scheme 9).
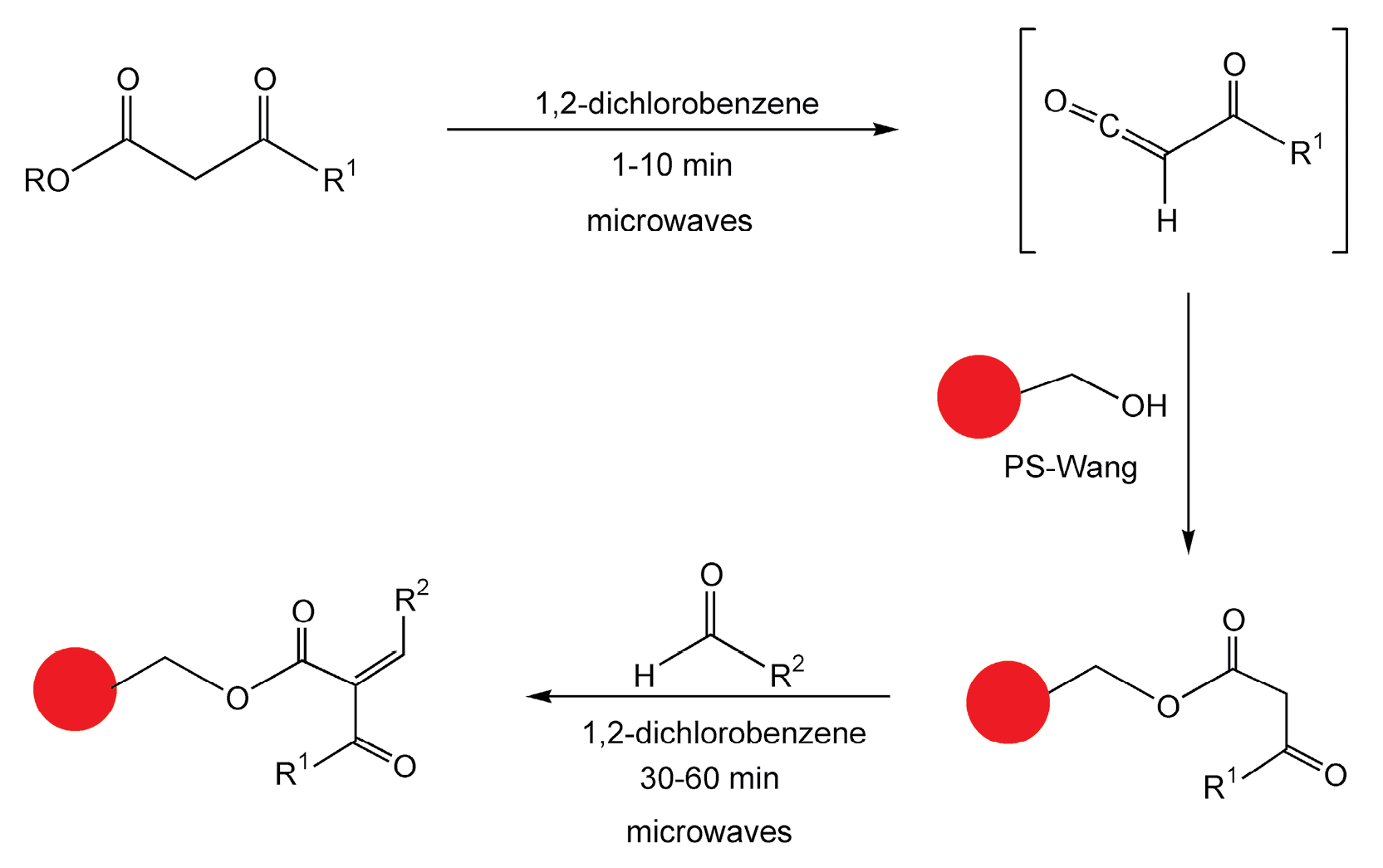
Scheme 9
Glass and Combs have also performed rapid parallel synthesis on solid-phase resins with microwave irradiation.31 They have examined the utility of a “safety catch” sulphonamide linker to produce large libraries of diverse amides and ureas. These safety catch linkers are highly stable through a given synthetic sequence until cleavage from the resin is necessary. The linker must first be activated before nucleophilic displacement of the substrate can occur. Conventional displacement of the substrate requires a very strong nucleophile and limits library diversity. The use of microwave irradiation allows for any nucleophile to displace the resin, including weak ones. Scheme 10 shows a facile biaryl urea synthesis, which includes a Suzuki coupling reaction, linker activation via alkylation, and subsequent cleavage with diisopropyl amine (DIPA).
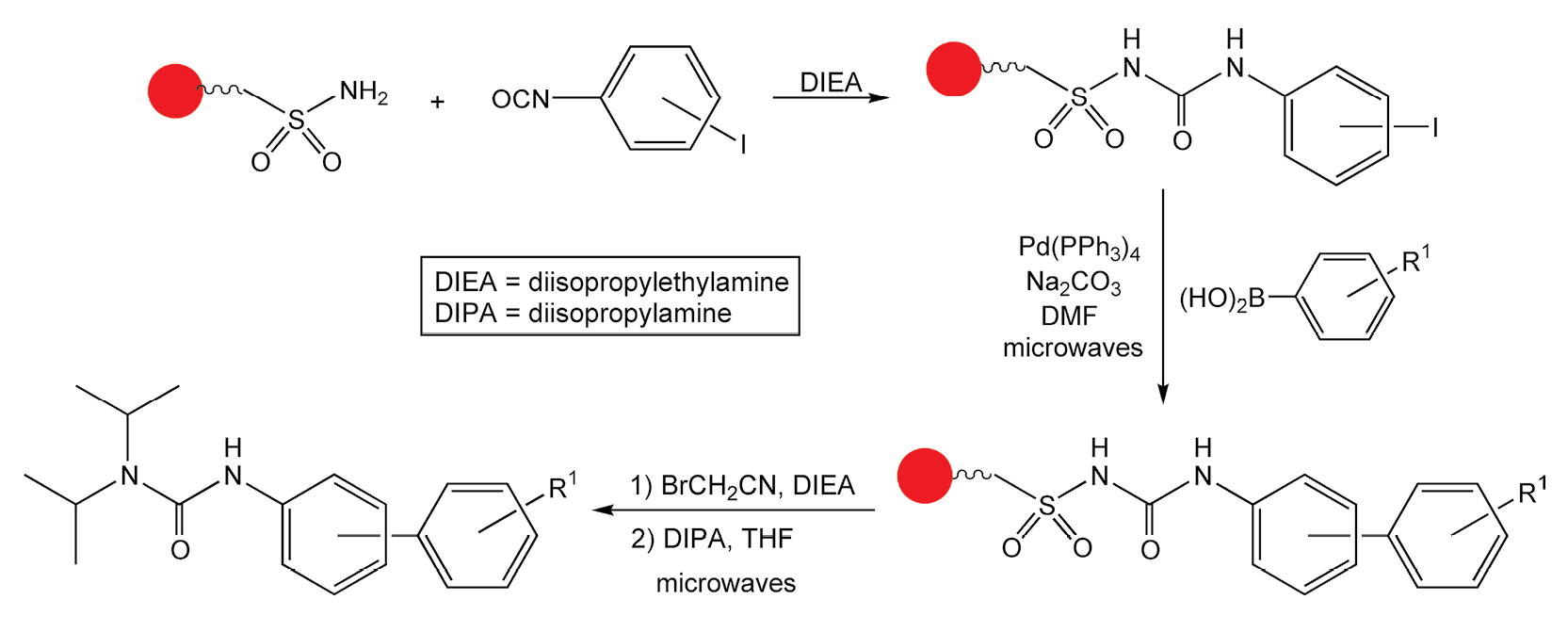
Scheme 10
Microwave-assisted, solid-phase syntheses are not restricted to spherical bead polymer resins. Scharn et al. used a planar cellulose membrane to synthesize a parallel library of 8000 1,3,5-triazines via nucleophilic substitution reactions.40 The planar membrane is composed of an array of “spots” that are individually derivatized. Scheme 11 illustrates how an amino-functionalized spot is doped with cyanuric chloride and then diversified with different amines by microwave heating. The second nucleophilic substitution requires five hours of thermal heat for completion, but with microwave irradiation, an entire library can be synthesized in six minutes.
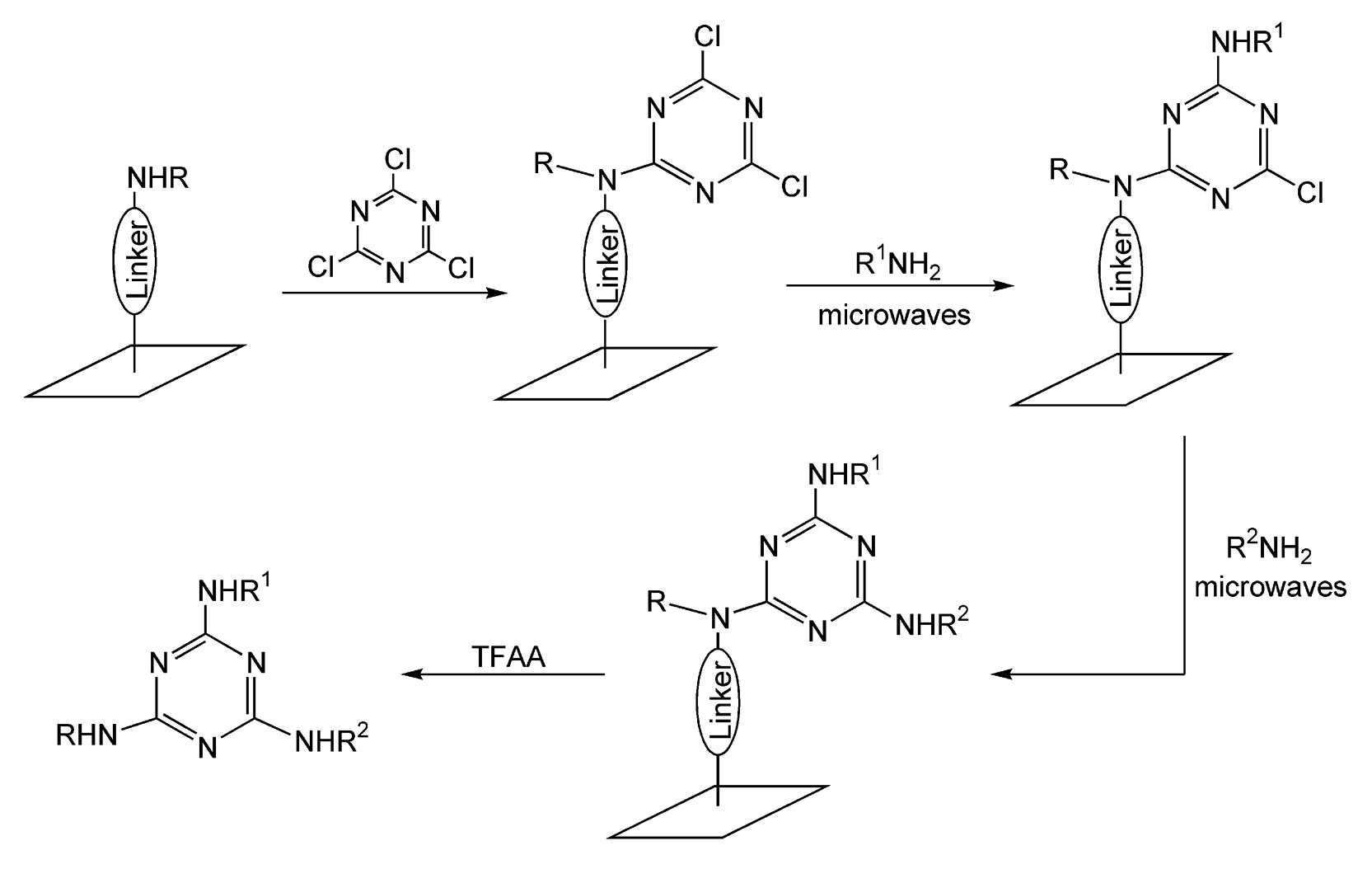
Scheme 11
Solvent Free (Neat) reactions
Reactions performed in a solvent-free environment are becoming more prevalent in organic chemistry. An increasing need for less hazardous reaction conditions and environmentally safe procedures, or green chemistry, has led chemical synthesis in this direction. Microwave irradiation has been used extensively in solvent-free reactions.5,8,16,20,43-181 There are three main types of solvent free reactions: reaction mixtures adsorbed onto mineral oxides, phase transfer catalysis (PTC), and neat reactions. This section will identify and provide examples for each type. For an overview of a wider range of different chemical transformations that can be performed solventless, the reader should consult Chapter 4, as there are over 100 additional solvent-free references.
An increasingly popular solvent-free method is to adsorb reagents onto mineral oxides. The reagent is first dissolved in an appropriate volatile solvent. After the mineral oxide (alumina, silica gel, clay, or zeolites) is added, the solvent is removed by evaporation. The impregnated solid support is then irradiated with microwaves in “dry media”. Upon completion of the reaction, a solvent is added to extract the product(s) from the support. Choice of solid support depends on the type of reaction a chemist is going to perform. Alumina can act as a base, but if a stronger one is needed, potassium fluoride on alumina is extremely basic. Silica gel naturally acts as a weak acid, while some of the montmorillonite clays provide acidities near sulfuric and nitric acids. As a whole, this solid-state application will greatly reduce the amount of solvent used that eventually needs to be properly disposed of and will minimize potentially hazardous reaction conditions.
Kidwai and co-workers have done extensive research in solvent-free reaction chemistry.37,104-113,276,290 Scheme 12 shows an example of a microwave-enhanced synthesis to N-acylated cephalosporin derivatives.37 Cephalosporanic acid and a heterocyclic carboxylic acid were adsorbed onto basic alumina and irradiated with microwaves for 2 minutes to yield the antibacterials in 82-93% yield. With thermal heat, this reaction can take anywhere from two to six hours and provides much lower yields. Another reaction performed on basic alumina is shown in Scheme 13.105,113 Barbituric and thiobarbituric acid derivatives are adsorbed onto the alumina with substituted arylmercuric chlorides to yield biologically active fungicides.
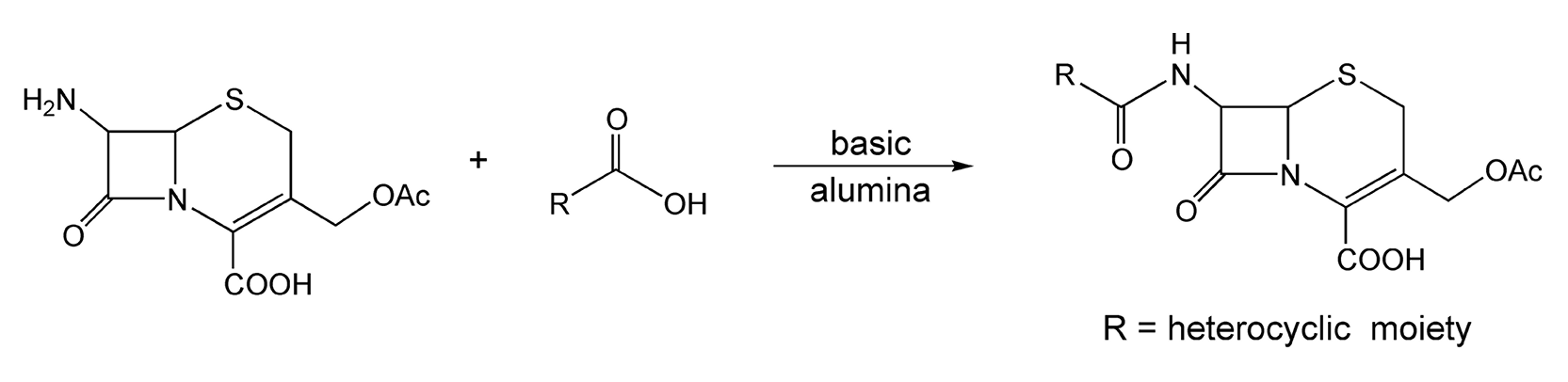
Scheme 12
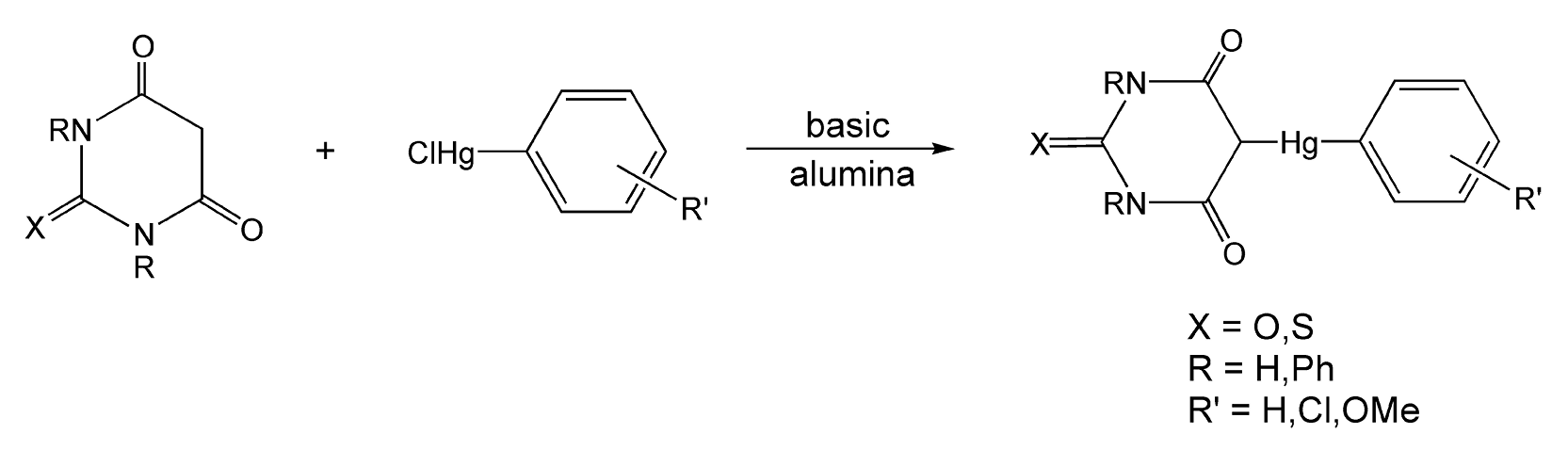
Scheme 13
Kabalka and co-workers have also explored solventless, microwave-enhanced reactions on dry media.99-101,628-629 Sonogashira coupling reactions are a palladium-catalyzed reaction between terminal alkynes and an aryl halide. These reactions typically employ a solvent and an amine, which produce environmental burdens. Scheme 14 illustrates a Sonogashira coupling that was performed on potassium fluoride/alumina doped with a palladium/copper iodide/triphenylphosphine mixture. The arylalkynes were synthesized in very high yields (82-97%).99 Another type of coupling reaction that can be performed in a solvent-free environment is Glaser coupling. This copper-catalyzed coupling of two terminal alkynes produces diacetylene derivatives, which are very important in the polymer and material science industries. Phenylacetylene and copper chloride on potassium fluoride/alumina, coupled with microwave irradiation, give diphenylbutadiyne in a 75% product yield (Scheme 15).
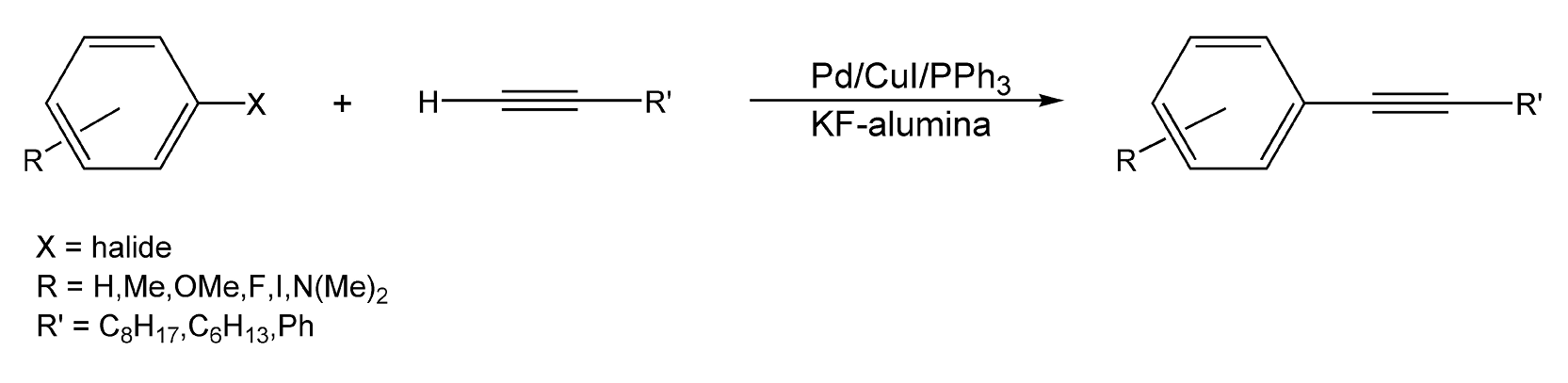
Scheme 14
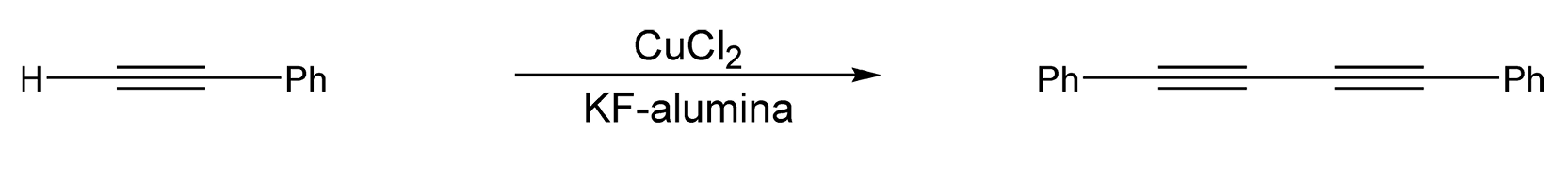
Scheme 15
The Baylis-Hillman reaction is an important carbon–carbon bond forming reaction that forms multifunctional molecules. In this reaction, an aldehyde reacts with an electron deficient alkene to yield allylic alcohol derivatives. Isomerization of acetylated Baylis Hillman adducts will yield (E)-trisubstituted alkenes, which are often difficult to synthesize. Microwave irradiation of the functionalized acetates on montmorillonite K10 clay yields trisubstituted alkenes in 13 minutes (Scheme 16).130 The clay acts as a catalyst, since only starting material is recovered in its absence.
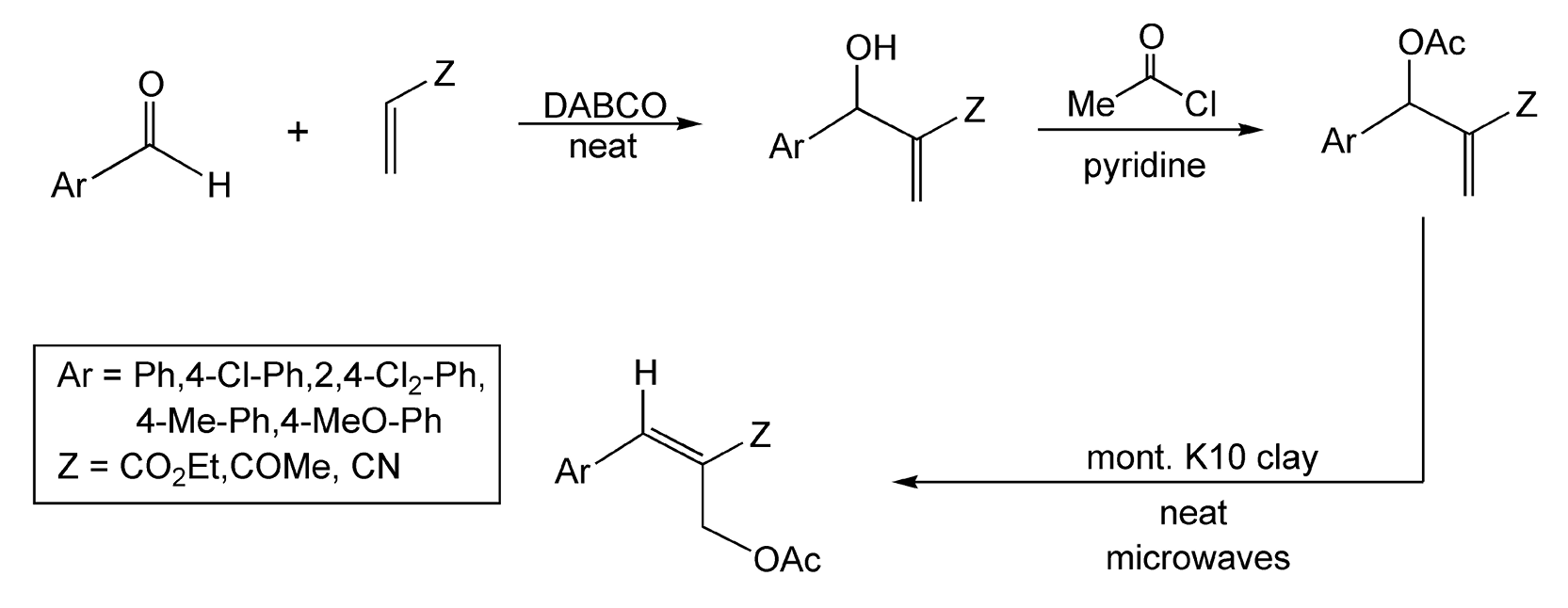
Scheme 16
Another pioneer in microwave-assisted solvent-free reactions is Andre Loupy.5,8,45-63,297,310,351,416,425,439,472,491,501,505-507, 522,568,590, 607,657,708,709 One important reaction that is used frequently in natural product syntheses is the Beckmann rearrangement. This reaction rearranges ketoximes to amides or lactams in the presence of acid. Traditionally, very strong acids are used to promote the rearrangement. Loupy and co-workers have performed facile Beckmann rearrangements on montmorillonite K10 clay under microwave irradiation in high yields (68-96%) (Scheme 17).60 Another microwave reaction performed by Loupy et al. in a solventless environment is carbohydrate glycosylation. Scheme 18 illustrates the glycosylation of peracetylated D-glucopyranose with decanol.54
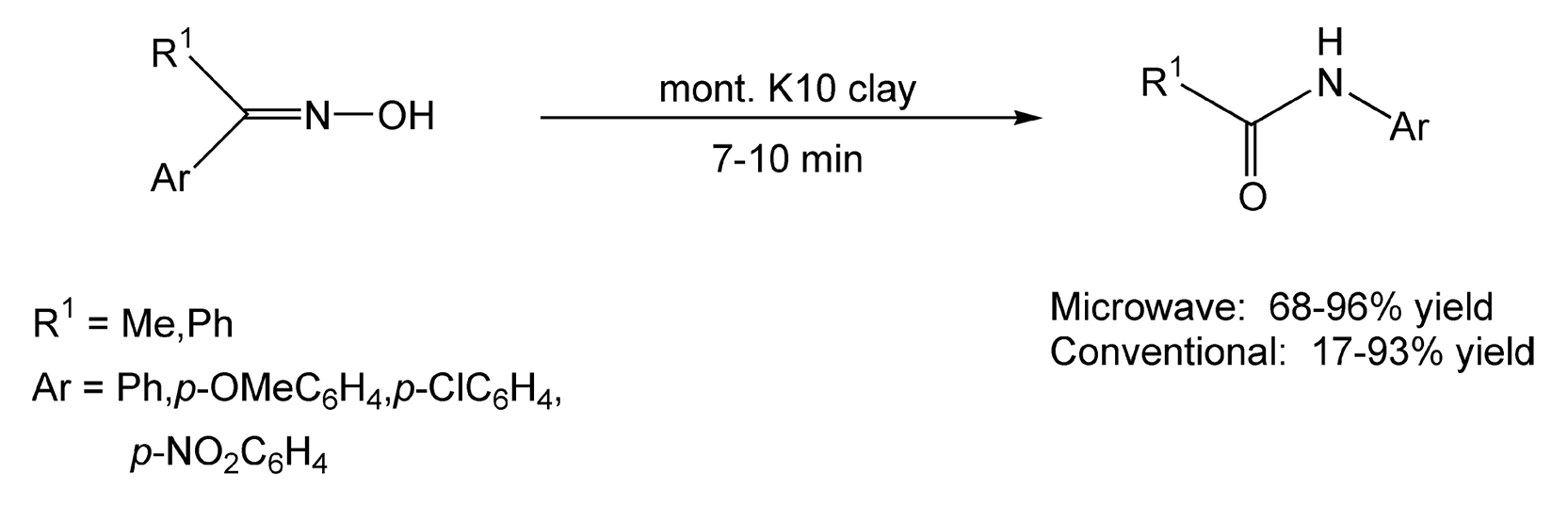
Scheme 17
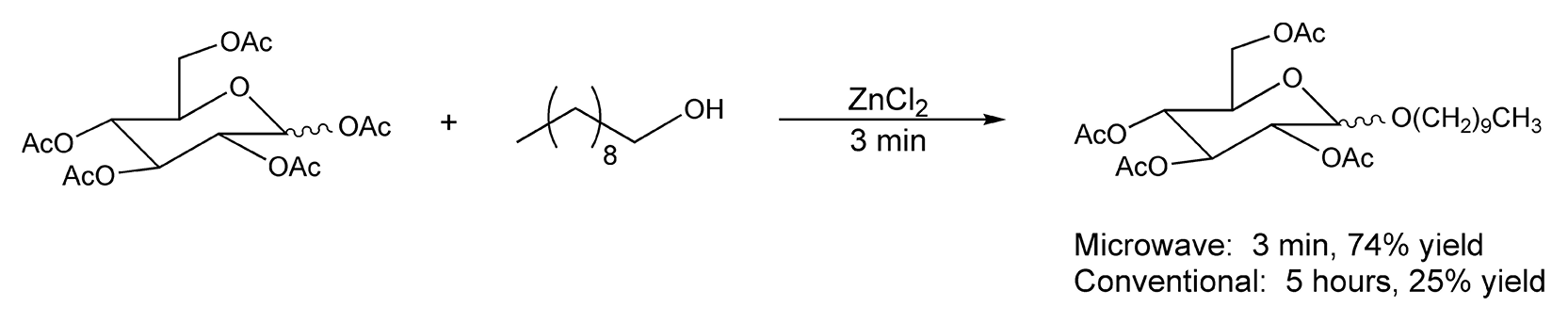
Scheme 18
Varma and co-workers have performed extensive research on microwave-assisted, solvent-free reactions in numerous areas including oxidations, reductions, protections, deprotections, and condensations.20,84-98 Many of these are discussed in Chapter 4 and include additional references. Another area of interest is the enamine-mediated approach to isoflav-3-ene synthesis. Enamines are traditionally synthesized via azeotropic removal of water and usually require an initial acid catalyst. Scheme 19 shows a microwave-enhanced, solvent-free, one-pot synthesis to isoflav-3-ene derivatives, which takes place in only seven minutes.98 An efficient microwave-induced tetrahydroquinolone synthesis effected on clay is completed in only two minutes (Scheme 20).96
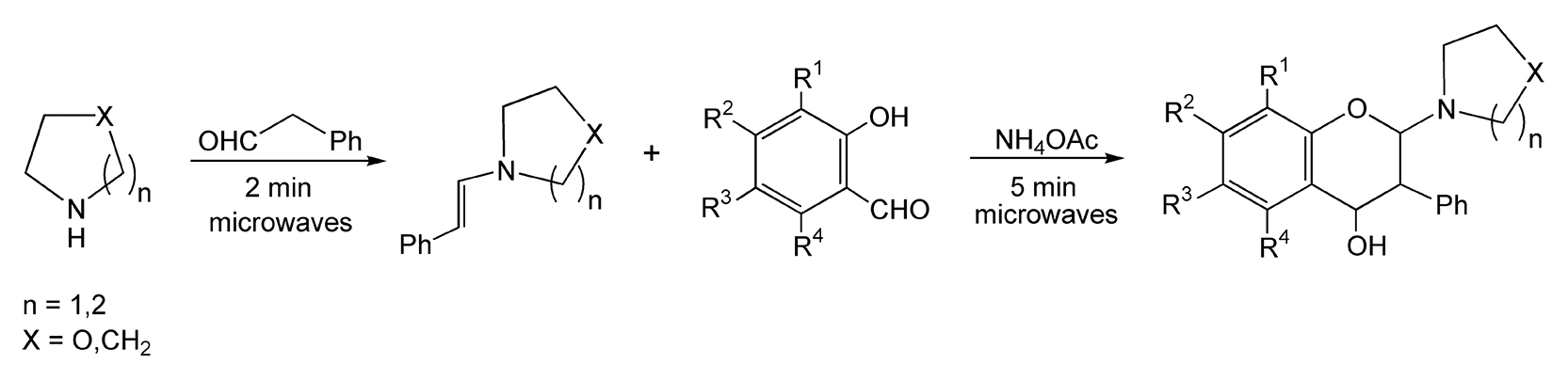
Scheme 19
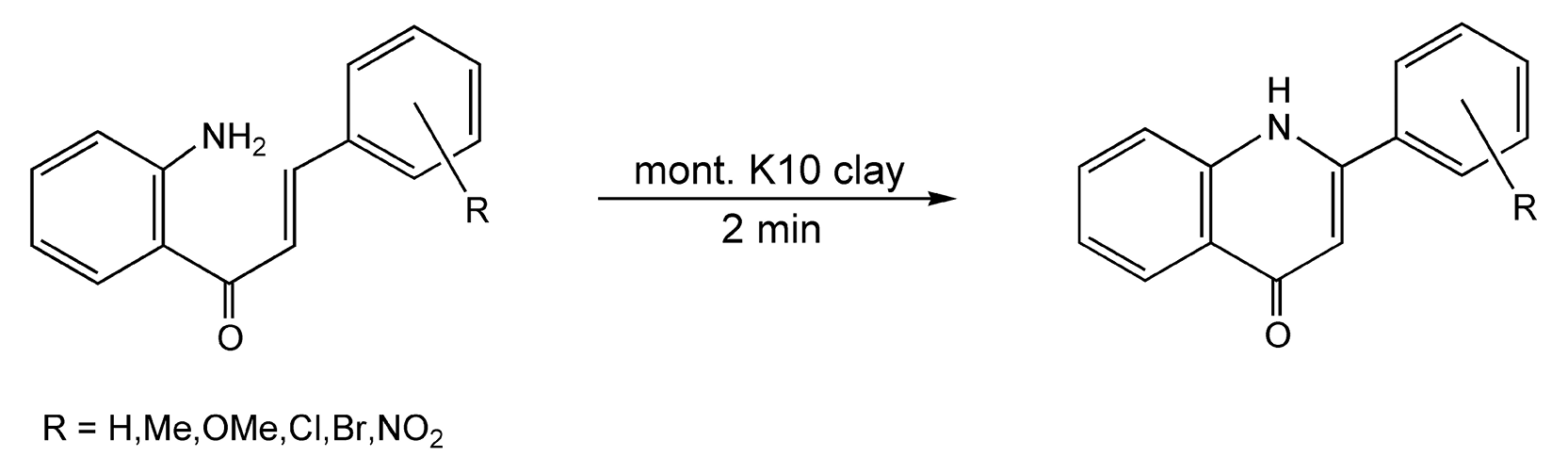
Scheme 20
Didier Villemin is yet another researcher who has examined reactions in dry media extensively. Metallophthalocyanines have become important molecules in the material science industries, as they are stable to strong acids and bases, as well as high temperatures. Traditional synthetic routes to phthalocyanines require long reaction times and very high temperatures. Villemin and co-workers have performed one-step metallophthalocyanine syntheses on clay, zirconium phosphate, and encapsulated in zeolite via microwave irradiation (Scheme 21).132 These reactions were completed in only five minutes and in quantitative yields.
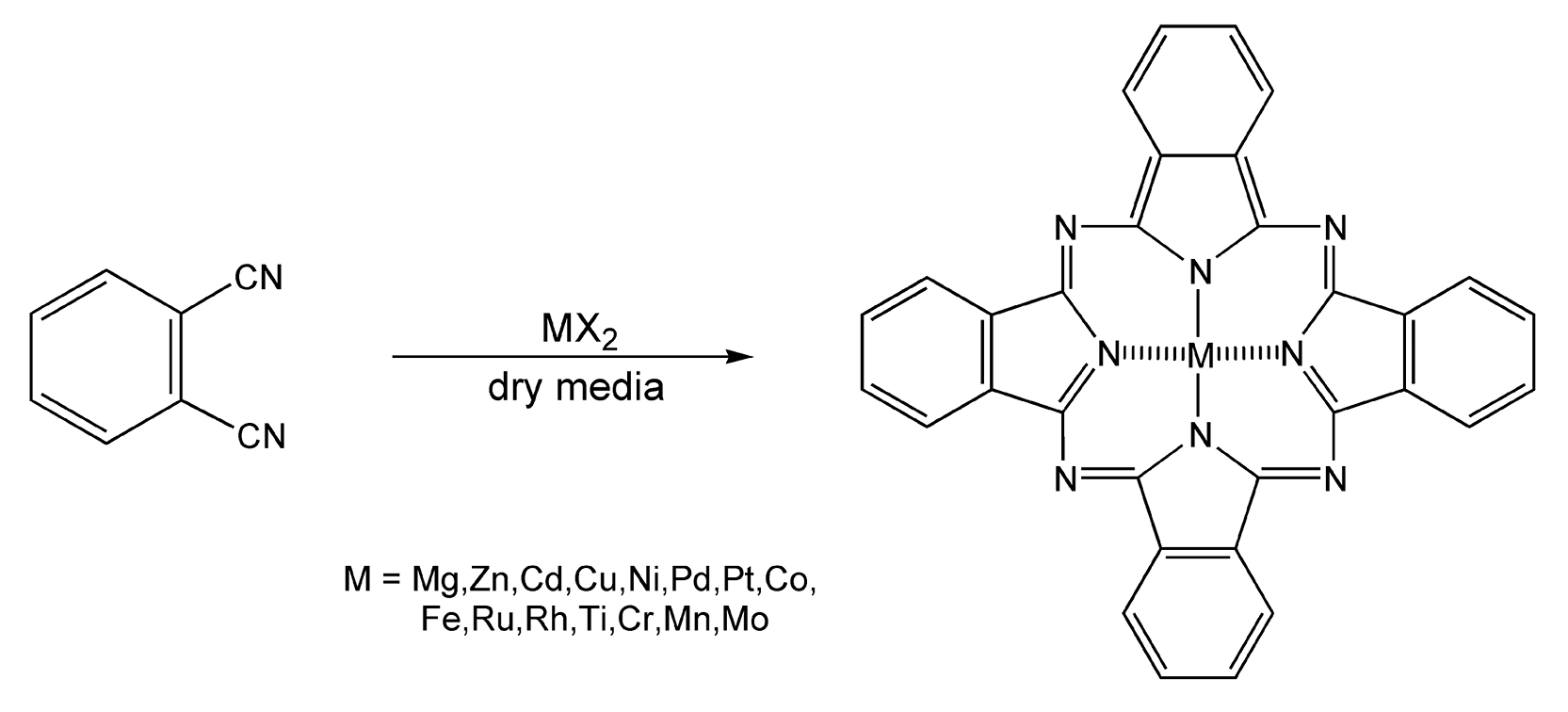
Scheme 21
Solid-liquid-phase transfer catalysis is another type of solvent-free reaction. With this method, a reagent acts as both a reactant and an organic phase. Microwave irradiation has been used extensively in these types of reactions.8,50,66,351,472,483,490,491,505,593 An inexpensive and useful phase transfer catalyst (PTC) is polyethylene glycol (PEG). Medium to high molecular weight PEG is a solid at room temperature, but at 50 °C, it melts to become a liquid. At temperatures above 50 °C, derivatized PEG can be used as a soluble polymeric support in the solution phase, but when cooled to room temperature, it becomes solid and provides for simple purification. Scheme 22 exhibits a PEG-supported alkyl-ation of a Schiff base to aminoacid derivatives under microwave irradiation in 75-98% yield.166
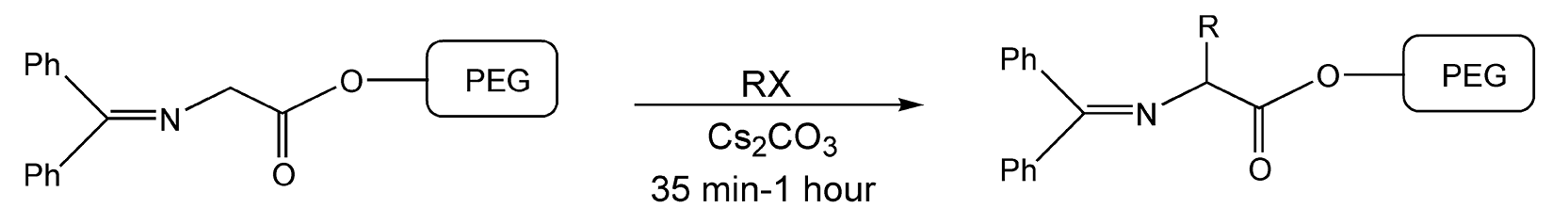
Scheme 22
Another useful PTC for microwave-assisted reactions is poly(styrene-co-allyl alcohol) (Ps-OH). This support possesses the properties of both PEG and polystyrene. Vanden Eynde and Rutot rapidly synthesized heterocyclic compounds via supported β-keto esters, with the first step only taking five minutes (Scheme 23).41 The parent polymer can be regenerated from the resulting acylated polymer by saponification.
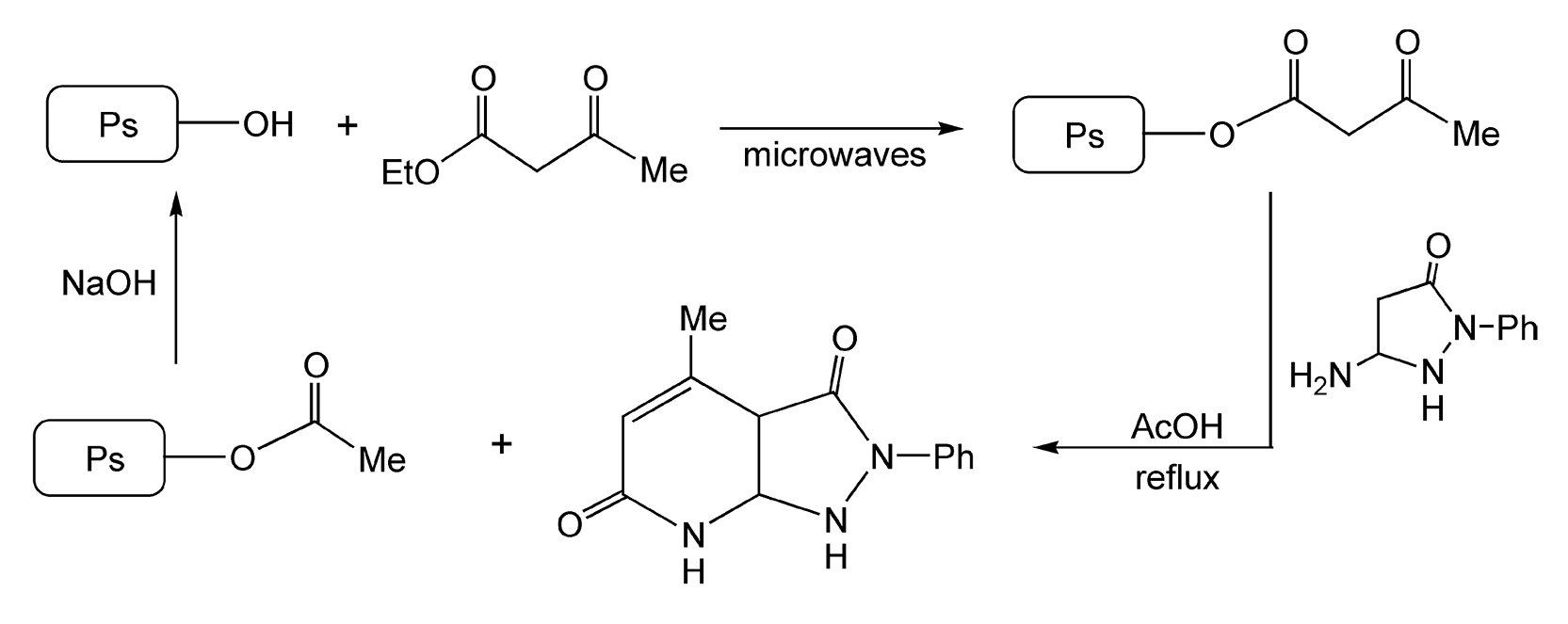
Scheme 23
Andre Loupy has also done some interesting research involving phase transfer catalysis coupled with microwave irradiation.8,50,62,351,472,491,505,593 β-Elimination of halogenated precursors, with potassium t-butoxide/tetrabutylammonium bromide (KOtBu/TBAB) as the PTC, provides a new route to ketene O,O- and S,S-acetals (Scheme 24).62 Compared to both conventional and ultrasonic methods, microwave irradiation produced much larger product yields.
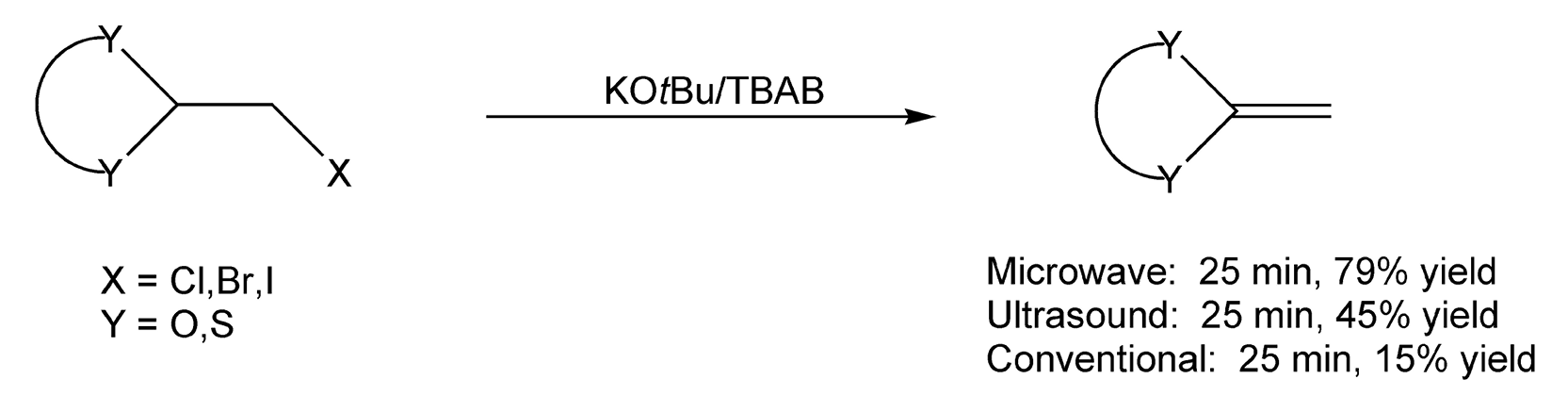
Scheme 24
Another area of interest includes the synthesis of furan diethers. These types of compounds constitute a large percentage of the derivatives that make up biomass, a renewable source of natural products. Loupy and co-workers developed two methods of microwave-assisted phase transfer catalysis for furan synthesis, solid-liquid PTC (solid KOH and Aliquat 336) and liquid-liquid PTC (aqueous KOH and Aliquat 336).351 Scheme 25 shows the reaction between 2,5-furandimethanol and an alkyl halide by both PTC methods. Phase transfer catalysis can also benefit the reaction between furfuryl alcohol and a dihalide (Scheme 26).
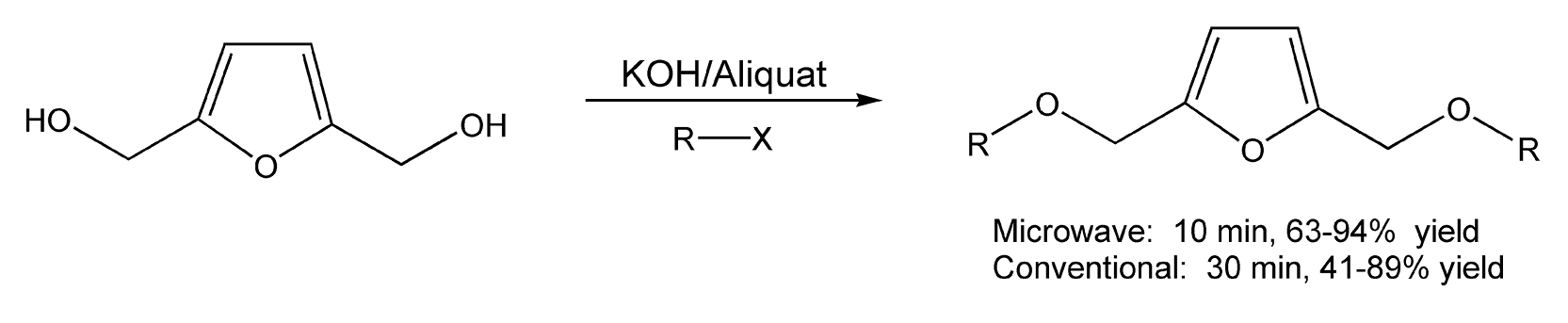
Scheme 25
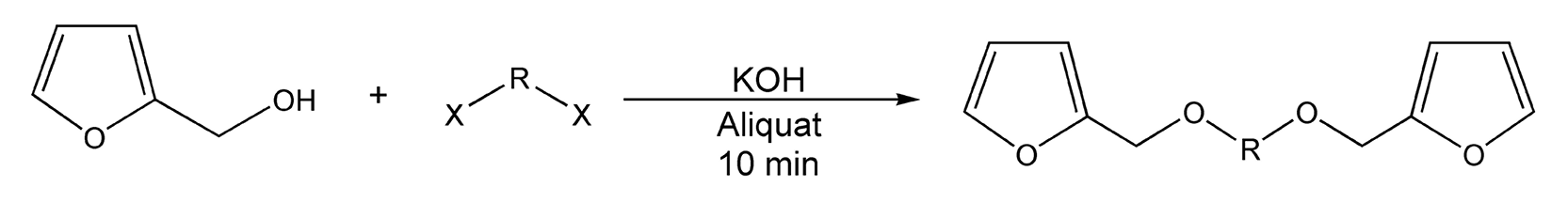
Scheme 26
Performing a reaction neat under microwave irradiation is the third type of solvent-free reaction. With this method, neither a mineral oxide nor a PTC is used, and the liquid or solid reagents are used directly from their containers with no dilutions. One interesting neat reaction utilizes Lawesson’s reagent, which transforms a carbonyl moiety into its thio analog. Scheme 27 exhibits the microwave-induced conversion of amides to thioamides in six minutes.88,165 An additional microwave example converts coumarins and other lactones to their thio derivatives in only 3 minutes with quantitative product yields (Scheme 28).88
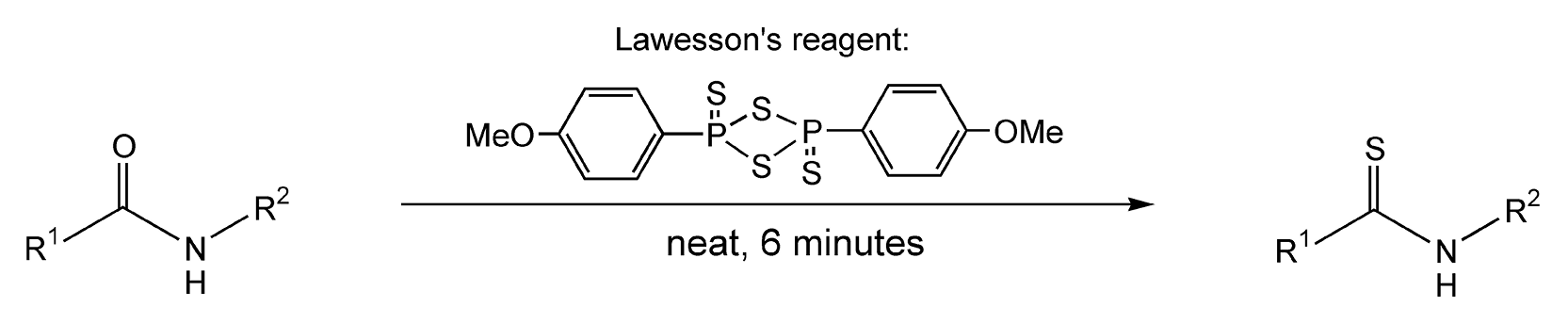
Scheme 27
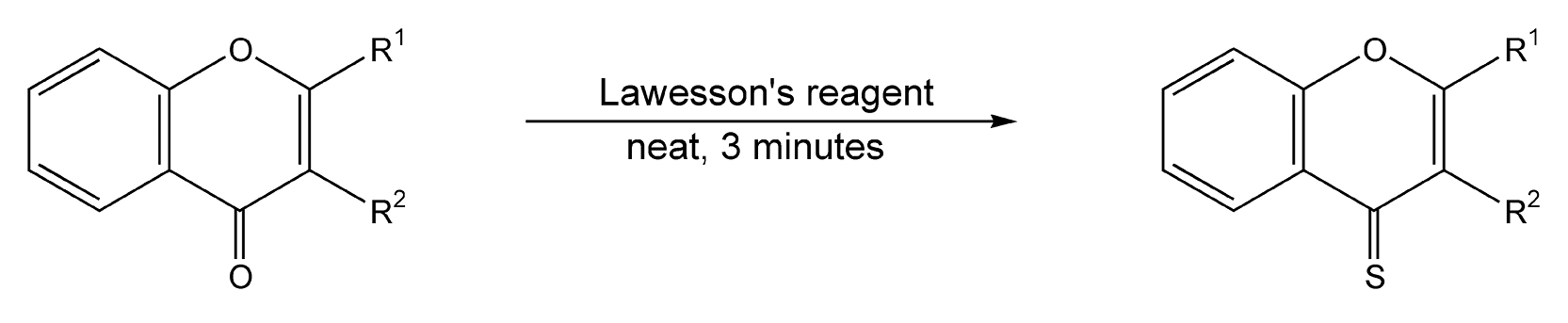
Scheme 28
Substituted 2-oxazolines are important heterocyclic intermediates used in drug discovery. Classical syntheses of these compounds require high temperatures, azeotropic water removal, and multi-step procedures. With microwave irradiation, 2-oxazolines are synthesized from the cyclodehydration reaction between a carboxylic acid and α,α,α-tris(hydroxymethyl) methyl amine without any solvent or solid support in 2-5 minutes (Scheme 29).57
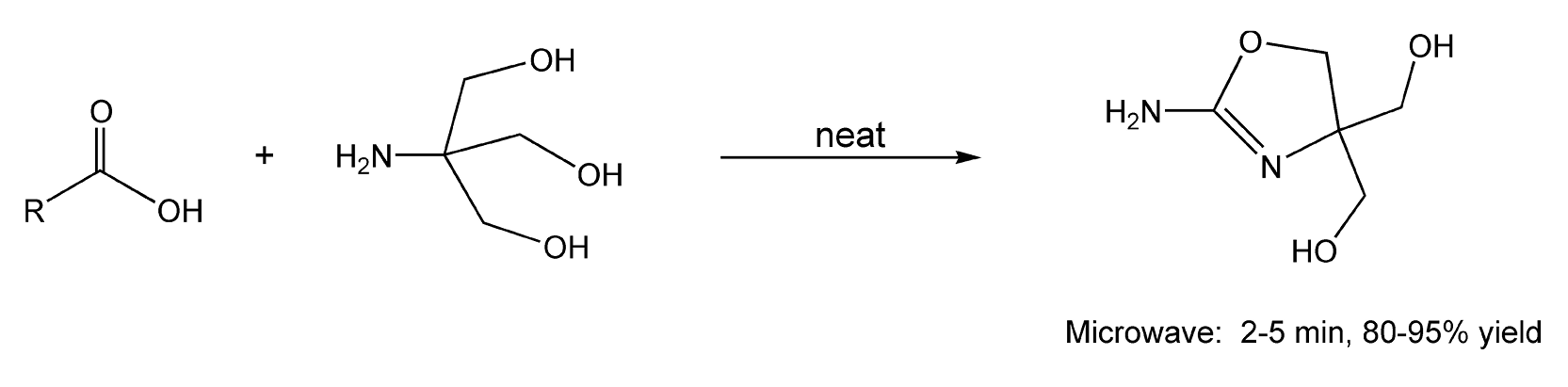
Scheme 29
Both Diels-Alder and 1,3-dipolar cycloadditions benefit from microwave-assisted neat conditions, as they require long reaction times and very high thermal temperatures. In a solventless environment, vinylpyrazoles react with substituted alkynes to yield non-aromatic cycloadducts via microwave irradiation in 15 minutes (Scheme 30).214 Schemes 31 and 32 illustrate successful 1,3-dipolar cycloadditions that yielded heterocycles in very high product yields.8,364
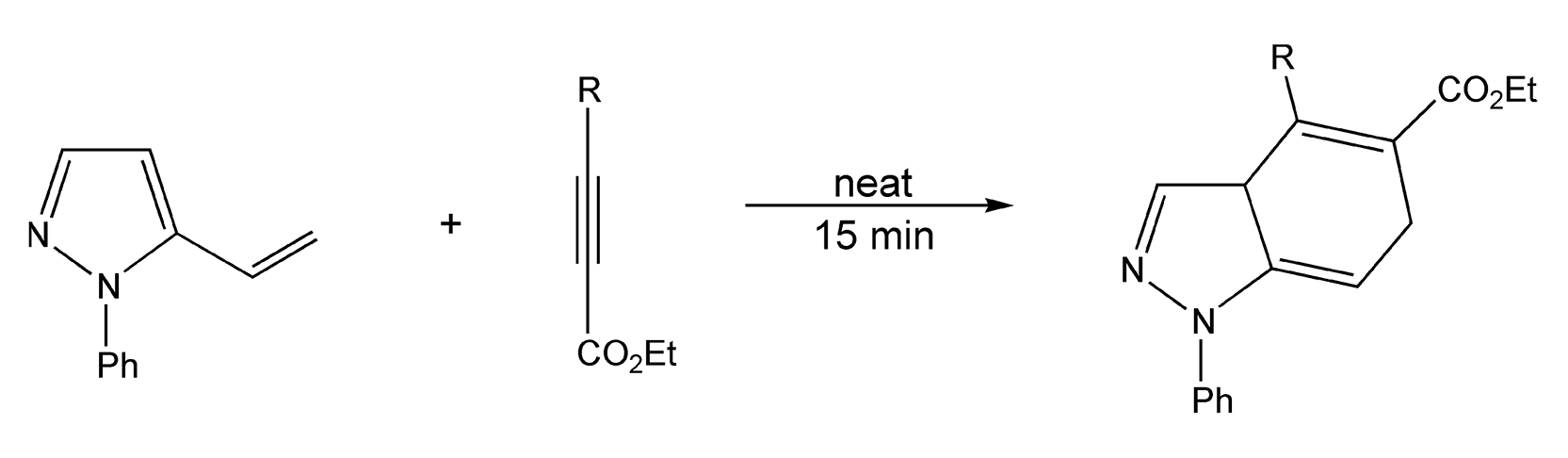
Scheme 30
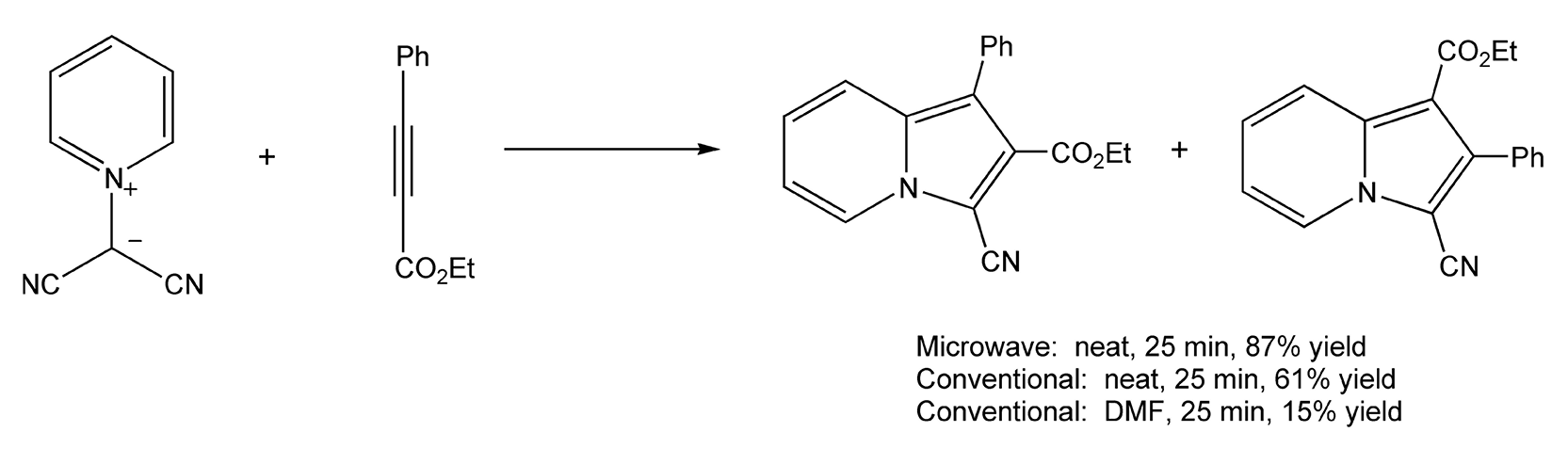
Scheme 31
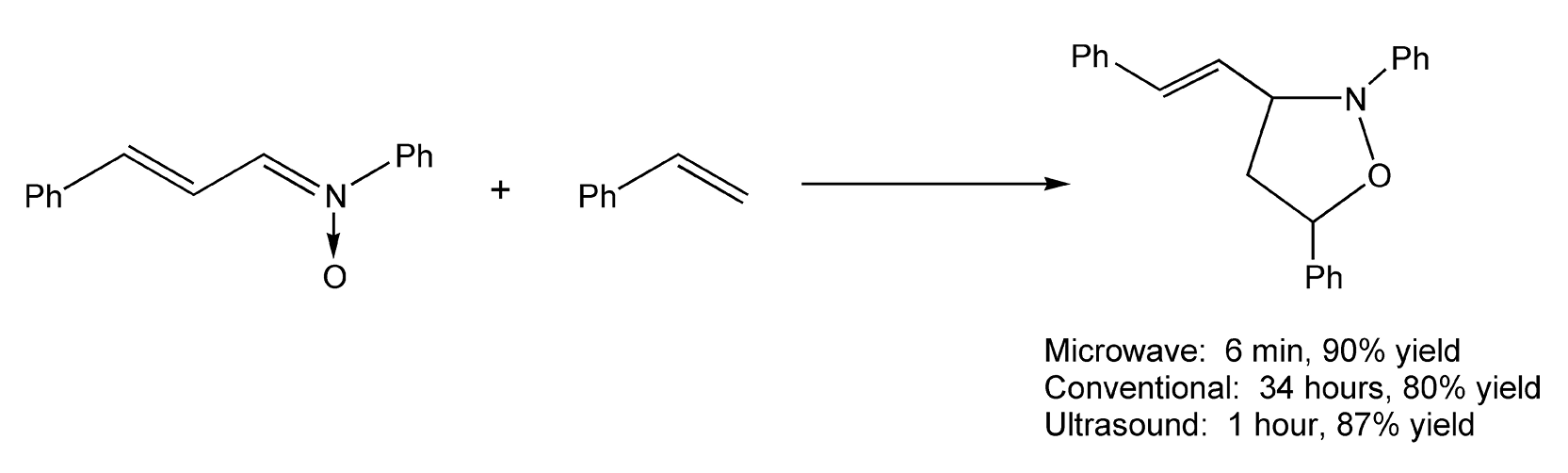
Scheme 32
Thus, the two main types of conditions used for chemical reactions, those run in the presence of solvent and those run in a solventless environment, are equally important and both can benefit from microwave heating. We have seen that microwave irradiation is not only applicable to standard homogeneous reaction mediums, but to solid-phase systems as well. Most synthetic methods can be executed by at least one of these systems. In conjunction with the following synthesis chapter, a chemist can now develop optimal and efficient synthetic routes.
Instruments
5. Jun, C.H.; Chung, J.H.; Lee, D.Y.; Loupy, A.; Chatti, S. “Solvent-free chelation-assisted intermolecular hydro-acylation: effect of microwave irradiation in the synthesis of ketone from aldehyde and 1-alkene by Rh(I) complex.” Tetrahedron Lett. 2001, 42, pp. 4803-05.
8. Loupy, A.; Petit, A.; Hamelin, J.; Texier-Boullet, F.; Jacquault, P.; Mathe, D. “New solvent-free organic synthesis using focused microwaves.” Synthesis 1998, 9, pp. 1213-34.
16. Kuhnert, N.; Danks, T.N. “Highly diastereoselective synthesis of 1,3-ozazolidines under thermodynamic control using focused microwave irradiation under solvent-free conditions.” Green Chem. 2001, 3, pp. 68-70.
20. a) Varma, R.S.; Namboodiri, V.V. “An expeditious solvent-free route to ionic liquids using microwaves.” J. Chem. Soc., Chem. Commun. 2001, 7, pp. 643-44. b) Varma, R.S.; Namboodiri, V.V. “Solvent-free preparation of ionic liquids using a household microwave oven.” Pure Appl. Chem. 2001, 73, pp. 1309-14.
27. Yu, H.M.; Chen, S.T.; Chiou, S.H.; Wang, K.T. “Determination of amino acids on Merrifield resin by microwave hydrolysis.” J. Chromatogr. 1988, 456, pp. 357-62.
28. Yu, H.M.; Chen, S.T.; Wang, K.T. “Enhanced coupling efficiency in solid-phase peptide synthesis by microwave irradiation.” J. Org. Chem. 1992, 57, pp. 4781-84.
29. Strohmeier, G.A.; Kappe, C.O. “Rapid parallel synthesis of polymer-bound enones utilizing microwave-assisted solid-phase chemistry.” J. Comb. Chem. 2002, 4, in press.
30. Lew, A.; Krutzik, P.O.; Hart, M.E.; Chamberlin, A.R. “Increasing rates of reaction: microwave-assisted organic synthesis for combinatorial chemistry.” J. Comb. Chem. 2002, 4, in press.
31. Glass, B.M.; Combs, A.P. “Rapid parallel synthesis utilizing microwave irradiation.” Fifth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5) 2001, E0027 (www.mdpi.net).
32. Kaboudin, B.; Balakrishna, M.S. “Surface-mediated solid phase reactions: microwave assisted Arbuzov rearrangement on the solid surface.” Synth. Commun. 2001, 31, pp. 2773-76.
33. Kaiser, N.F.K.; Hallberg, A.; Larhed, M. “Solid phase carbonylation in fast microwave-mediated combinatorial drug discovery.” Fifth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5) 2001, E0007 (www.mdpi.net).
34. Kappe, C.O. “Speeding up solid-phase chemistry by microwave irradiation: a tool for high-throughput synthesis.” Amer. Lab. 2001, 33, pp. 13-19.
35. Stadler, A.; Kappe, C.O. “High-speed couplings and cleavages in microwave-heated, solid-phase reactions at high temperatures.” Eur. J. Org. Chem. 2001, pp. 919-25.
36. Hajipour, A.R.; Mallakpour, S.E.; Afrousheh, A. “One-pot and simple reaction for the synthesis of alkyl p-toluenesulfinate esters under solid-phase conditions.” Phosphorus Sulfur Silicon Relat. Elem. 2000, 160, pp. 67-75.
37. Kidwai, M.; Misra, P.; Bhushan, K.R.; Saxena, R.K.; Singh, M. “Microwave-assisted solid-phase synthesis of cephalosporin derivatives with antibacterial activity.” Monatsh. Chem. 2000, 131, pp. 937-43.
38. Kumar, H.M.S.; Anjaneyulu, S.; Reddy, B.V.S.; Yadav, J.S. “Microwave-assisted rapid Claisen rearrangement on solid phase.” Synlett. 2000, 8, pp. 1129-30.
39. Stadler, A.; Kappe, C.O. “Solid phase coupling of benzoic acid to Wang resin: a comparison of thermal versus microwave heating.” Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4) 2000, B0002 (www.mdpi.net).
40. Scharn, D.; Wenschuh, H.; Reineke, U.; Schneider-Mergener, J.; Germeroth, L. “Spatially addressed synthesis of amino- and amino-oxy-substituted 1,3,5-triazine arrays on polymeric membranes.” J. Comb. Chem., 2000, 2, pp. 361-69.
41. Vanden Eynde, J.J.; Rutot, D. “Microwave-mediated derivatization of poly(styrene-co-allyl alcohol), a key step for the soluble polymer-assisted synthesis of heterocycles.” Tetrahedron 1999, 55, pp. 2687-94.
42. Yu, A.M.; Zhang, Z.P.; Yang, H.Z.; Zhang, C.X.; Liu, Z. “Wang resin bound addition reactions under microwave irradiation.” Synth. Commun. 1999, 29, 1595-99.
43. Gupta, M.; Paul, S.; Gupta, R. “Synthesis of 1,4-dithiocarbonyl piperazines under microwave irradiation in solvent-free conditions.” Synth. Commun. 2001, 31, pp. 53-59.
44. Paul, S.; Gupta, M.; Gupta, R. “Vilsmeier reagent for formylation in solvent-free conditions using microwaves.” Synlett. 2000, 8, pp. 1115-18.
45. Paul, S.; Gupta, M.; Gupta, R.; Loupy, A. “Microwave assisted synthesis of 1,5-disubstituted hydantoins and thiohydantoins in solvent-free conditions.” Synthesis 2002, 1, pp. 75-78.
46. Paul, S.; Gupta, R.; Loupy, A.; Rani, B.; Dandia, A. “Dry media synthesis of 4H-1,4-benzothiazines under microwave irradiation using basic alumina as solid support.” Synth. Commun. 2001, 31, pp. 711-17.
47. Paul, S.; Gupta, M.; Gupta, R.; Loupy, A. “Microwave assisted solvent-free synthesis of pyrazolo[3,4-b]quinolines and pyrazolo[3,4-c]pyrazoles using p-TsOH.” Tetrahedron Lett. 2001, 42, pp. 3827-29.
48. Genta, M.T.; Villa, C.; Mariani, E.; Loupy, A.; Petit, A.; Rizzetto, R.; Mascarotti, A.; Morini, F.; Ferro, M. “Microwave-assisted preparation of cyclic ketals from a cineole ketone as potential cosmetic ingredients: solvent-free synthesis, odour evaluation, in vitro cytotoxicity, and antimicrobial assays.” Intl. J. Pharmaceutics 2002, 231, pp. 11-20.
49. Rodriguez, H.; Perez, R.; Suarez, M.; Lam, A.; Cabrales, N.; Loupy, A. “Alkylation of some pyrimidine and purine derivatives in the absence of solvent using microwave-assisted method.” Fifth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5) 2001, E0004 (www.mdpi.net).
50. Villa, C.; Genta, M.T.; Bargagna, A.; Mariani, E.; Loupy, A. “Microwave activation and solvent-free phase transfer catalysis for the synthesis of new benzylidene cineole derivatives as potential UV sunscreens.” Green Chem. 2001, 3, pp. 196-200.
51. Cleophax, J.; Liagre, M.; Loupy, A.; Petit, A. “Application of focused microwaves to the scale-up of solvent-free organic reactions.” Org. Process Res. Dev. 2000, 4, pp. 498-504.
52. Loupy, A.; Régnier, S. “ Solvent-free microwave-assisted Beckmann rearrangement of benzaldehyde and 2-hydroxyacetophenone oximes.” Tetrahedron Lett. 1999, 40, pp. 6221-24.
53. Gelo-Pujic, M.; Guibe-Jampel, E.; Loupy, A.; Trincone, A. “Enzymatic glycosidation in dry media under microwave irradiation.” J. Chem. Soc., Perkin Trans. 1 1997, pp. 1001-02.
54. Limousin, C.; Cleophaz, J.; Petit, A.; Loupy, A.; Lukacs, G. “Solvent-free synthesis of decyl D-glycopyranosides: under focused microwave irradiation.” J. Carbohydrate Chem. 1997, 16, pp. 327-42.
55. Sotelo, E.; Mocelo, R.; Suarez, M.; Loupy, A. “Synthesis of polyfunctional pyridazine derivatives using a solvent-free microwave assisted method.” Synth. Commun. 1997, 27, pp. 2419-23.
56. Loupy, A.; Pigeon, P.; Ramdani, M. “Synthesis of long chain aromatic esters in a solvent-free procedure under microwaves.” Tetrahedron 1996, 52, pp. 6705-12.
57. Marrero-Terrero, A.L.; Loupy, A. “Synthesis of 2-oxazolines from carboxylic acids and α,α,α-tris(hydroxy-methyl)methylamine under microwaves in solvent-free conditions.” Synlett. 1996, 3, pp. 245-46.
58. Perez, E.; Sotelo, E.; Loupy, A.; Mocelo, R.; Suarez, M.; Perez, R.; Autie, M. “An easy and efficient microwave-assisted method to obtain 1-(4-bromophenacyl)azoles in dry media.” Heterocycles 1996, 47, pp. 539-43.
59. Suarez, M.; Loupy, A.; Perez, E.; Moran, L.; Gerona, G.; Morales, A.; Autie, M. “An efficient procedure to obtain hexahydroquinomeines and unsymmetrical 1,4-dihydropyridines using solid inorganic supports and microwave activation.” Heterocycl. Commun. 1996, 2, pp. 275-80.
60. Bosch, A.; de la Cruz, P.; Diez-Barra, E.; Loupy, A.; Langa, F. “Microwave assisted Beckmann rearrangement of ketoximes in dry media.” Synlett. 1995, 12, pp. 1259-60.
61. Loupy, A.; Petit, A.; Bonnet-Delpon, D. “Improvements in 1,3-dipolar cycloaddition of nitrones to flourinated dipolarophiles under solvent-free microwave activation.” J. Flourine Chem. 1995, 75, pp. 215-16.
62. Diaz-Ortiz, A.; Prieto, P.; Loupy, A.; Abenhaim, D. “A short and efficient synthesis of ketene O,O- and S,S-acetals under focused microwave irradiation and solvent-free conditions.” Tetrahedron Lett. 1996, 37, pp. 1695-98.
63. Diaz-Ortiz, A.; Diez-Barra, E.; de la Hoz, A.; Moreno, A.; Gomez-Escalonilla, M.J.; Loupy, A. “1,3-Dipolar cycloaddition of nitriles under microwave irradiation in solvent-free conditions.” Heterocycles 1996, 43, pp. 1021-30.
64. Diaz-Ortiz, A.; de la Hoz, A.; Langa, F. “Microwave irradiation in solvent-free conditions: an eco-friendly methodology to prepare indazoles, pyrazolopyridines and bipyrazoles by cycloaddition reactions.” Green Chem. 2000, 2, pp. 165-72.
65. Vaquez, E.; de la Hoz, A.; Elander, N.; Moreno, A.; Stone-Elander, S. “Microwave-assisted cyclocondensation under solvent-free conditions: quinoxaline-2,3-dione.” Heterocycles 2001, 55, pp. 109-13.
66. de la Cruz, P.; de la Hoz, A.; Font, L.M.; Langa, F.; Pérez-Rodríguez, M.C. “Solvent-free phase transfer catalysis under microwaves in fullerene chemistry. A convenient preparation of N-alkylpyrrolidino[60]fullerenes.” Tetrahedron Lett. 1998, 39, pp. 6053-56.
67. Balalaie, S.; Sharifi, A.; Ahangarian, B.; Kowsari, E. “Microwave enhanced synthesis of quinazolines in solvent-free condition.” Heterocycl. Commun. 2001, 7, pp. 337-40.
68. Balalaie, S.; Kowsari, E. “One-pot synthesis of N-substituted 4-aryl-1,4-dihydropyridines under solvent-free condition and microwave irradiation.” Fifth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5) 2001, E0026 (www.mdpi.net).
69. Balalaie, S.; Salimi, S.H.; Sharifi, A. “Solid state deoximation with zinc chlorochromate: regeneration of carbonyl compounds.” Indian J. Chem., Sect. B 2001, 40, pp. 1251-52.
70. Balalaie, S.; Golizeh, M. “Solvent-free organic synthesis on mineral supports using microwave irradiation.” Fifth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5) 2001, E0025 (www.mdpi.net).
71. Balalaie, S.; Hashtroudi, M.S.; Sharifi, A. “Microwave-assisted synthesis of 1,3,5-trialkyltetrahydro-1,3,5-triazin-2(1H)-ones and a 4-oxooxadiazinane in dry media.” J. Chem. Res. (S) 1999, pp. 392-93.
72. Ballini, R.; Bosica, G.; Fiorini, D. “Stereoselective preparation of (E)-ε-nitro-β,γ-unsaturated methyl esters: Amberlyst A 27, using microwave, as superior catalyst for the 1,6-conjugate addition of nitroalkanes to methyl 1,3-butadiene-1-carboxylate.” Tetrahedron Lett. 2001, 42, pp. 8471-73.
73. Chemat, F.; Poux, M. “Microwave assisted pyrolysis of urea supported on graphite under solvent-free conditions.” Tetrahedron Lett. 2001, 42, pp. 3693-95.
74. Danks, T.N.; Desai, B. “Microwave assisted chemistry using supported formates as reagents in organic chemistry.” Fifth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5) 2001, E0045 (www.mdpi.net).
75. Hajipour, A.R.; Mallakpour, S.E.; Imanzadeh, G. “An efficient and novel method for the synthesis of aromatic sulfones under solvent-free conditions.” Indian J. Chem. Sec. B 2001, 40, pp. 237-39.
76. Hajipour, A.R.; Mallakpour, S.E.; Mohammadpoor-Baltork, I.; Khoee, S. “An efficient and selective method for conversion of oximes and semicarbazones to the corresponding carbonyl compounds under solvent-free conditions.” Synth. Commun. 2001, 31, pp. 1187-94.
77. Hajipour, A.R.; Ghasemi, M. “A rapid and convenient synthesis of amides from aromatic acids and aliphatic amines in dry media under microwave irradiation.” Indian J. Chem. Sec. B 2001, 40, pp. 504-07.
78. Hajipour, A.R.; Mallakpour, S.E.; Afrousheh, A. “A convenient and mild procedure for the synthesis of alkyl p-toluenesulfinates under solvent-free conditions using microwave irradiation.” Tetrahedron 1999, 55, pp. 2311-16.
79. Heravi, M.M.; Tajbakhsh, M. “Solid state deoximation with clay supported potassium ferrate under microwave irradiation.” Phosphorus Sulfur Silicon Relat. Elem. 2001, 176, pp. 195-99.
80. Heravi, M.M.; Ajami, D.; Mohajerani, B.; Tajbakhsh, M.; Ghassemzadeh, M.; Tabar-Hydar, K. “Solid state desemicarbazonation on clayfen under microwave irradiation.” Monatsh. Chem. 2001, 132, pp. 881-83.
81. Heravi, M.M.; Rajabzadeh, G.; Rahimizadeh, M.; Bakavoli, M.; Ghassemzadeh, M. “Thiation of heterocycles using silica gel supported P2S5 under microwave irradiation in solventless system.” Synth. Commun. 2001, 31, pp. 2231-34.
82. Tajbakhsh, M.; Heravi, M.M.; Habibzadeh, S.; Ghassemzadeh, M. “Microwave-assisted eco-friendly cleavage of acetals using supported potassium ferrate.” Phosphorus Sulfur Silicon Relat. Elem. 2001, 176, pp. 151-55.
83. Tajbakhsh, M.; Heravi, M.M.; Habibzadeh, S. “Potassium ferrate supported on silica gel: a mild, efficient, and inexpensive reagent for oxidative deprotection of tetrahydropyranyl ethers in nonaqueous conditions.” Phosphorus Sulfur Silicon Relat. Elem. 2001, 176, pp. 191-94.
84. Jeselnik, M.; Varma, R.S.; Polanc, S.; Kocevar, M. “Catalyst-free reactions under solvent-free conditions: microwave-assisted synthesis of heterocyclic hydrazones below the melting points of neat reactants.” J. Chem. Soc., Chem. Commun. 2001, pp. 1716-17.
85. Jeselnik, M.; Varma, R.S.; Polanc, S.; Kocevar, M. “Solid-state synthesis of heterocyclic hydrazones using microwaves under catalyst-free conditions.” Fifth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5) 2001, E0014 (www.mdpi.net).
86. Varma, R.S. “Solvent-free accelerated organic syntheses using microwaves.” Pure Appl. Chem. 2001, 73, pp. 193-98.
87. Varma, R.S. “Expeditious solvent-free organic syntheses using microwave irradiation.” Green Chem. Synth. Proc. 2000, 767, pp. 292-312.
88. Varma, R.S.; Kumar, D. “Microwave-accelerated solvent-free synthesis of thioketones, thiolactones, thioamides, thionoesters, and thioflavonoids.” Org. Lett. 1999, 1, pp. 697-700.
89. Varma, R.S. “Solvent-free organic synthesis using supported reagents and microwave irradiation.” Green Chem. 1999, 1, pp. 43-55.
90. Varma, R.S.; Naicker, K.P. “Solvent-free synthesis of amides from non-enolizable esters and amines using microwave irradiation.” Tetrahedron Lett. 1999, 40, pp. 6177-80.
91. Varma, R.S. “Solvent-free synthesis of heterocyclic compounds using microwaves.” J. Heterocycl. Chem. 1999, 36, pp. 1565-71.
92. Vass, A.; Dudas, J.; Varma, R.S. “Solvent-free synthesis of N-sulfonylimines using microwave irradiation.” Tetrahedron Lett. 1999, 40, pp. 4951-54.
93. Varma, R.S.; Naicker, K.P. “Hydroxylamine on clay: a direct synthesis of nitriles from aromatic aldehydes using microwaves under solvent-free conditions.” Molecules Online 1998, 2, pp. 94-96.
94. Varma, R.S.; Naicker, K.P.; Liesen, P.J. “Microwave-accelerated crossed Cannizzaro reaction using barium hydroxide under solvent-free conditions.” Tetrahedron Lett. 1998, 39, pp. 8437-40.
95. Varma, R.S.; Naicker, K.P.; Liesen, P.J. “Selective nitration of styrenes with clayfen and clayan: a solvent-free synthesis of β-nitrostyrenes.” Tetrahedron Lett. 1998, 39, pp. 3977-80.
96. Varma, R.S.; Saini, R.K. “Microwave-assisted isomerization of 2’-aminochalcones on clay: an easy route to 2-aryl-1,2,3,4-tetrahydro-4-quinolones.” Synlett. 1997, 87, pp. 857-58.
97. Varma, R.S.; Dahiya, R.; Kumar, S. “Clay catalyzed synthesis of imines and enamines under solvent-free conditions using microwave irradiation.” Tetrahedron Lett. 1997, 38, pp. 2039-42.
98. Varma, R.S.; Dahiya, R. “An expeditious and solvent-free synthesis of 2-amino-substituted isoflav-3-enes using microwave irradiation.” J. Org. Chem. 1998, 63, pp. 8038-41.
99. Kabalka, G.W.; Pagni, R.M.; Wang, L.; Namboodiri, V. “Microwave-assisted, solventless Suzuki coupling reactions on palladium-doped alumina.” Green Chem. 2000, 2, pp. 120-22 and Fifth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5) 2001, E0029 (www.mdpi.net).
100. Kabalka, G.W.; Wang, L.; Namboodiri, V.; Pagni, R.M. “Rapid microwave-enhanced, solventless Sonogashira coupling reaction on alumina.” Tetrahedron Lett. 2000, 41, pp. 5151-54.
101. Kabalka, G.W.; Wang, L.; Pagni, R.M. “Microwave-enhanced Glaser coupling under solvent free conditions.” Synlett. 2001, 1, pp. 108-10.
102. Kaboudin, B.; Nazari, R. “A convenient and mild procedure for the preparation of α-ketophosphonates of 1-hydroxyphosphonates under solvent-free conditions using microwave.” Synth. Commun. 2001, 31, pp. 2245-50.
103. Kaboudin, B.; Nazari, R. “Microwave-assisted synthesis of 1-aminoalkylphosphonates under solvent-free conditions.” Tetrahedron Lett. 2001, 42, pp. 8211-13.
104. Kidwai, M.; Misra, P.; Bhushan, K.R. “Alumina-supported synthesis of thiadiazolyl thiazolothiones.” Synth. Commun. 2001, 31, pp. 817-22.
105. Kidwai, M. “Dry media reactions.” Pure Appl. Chem. 2001, 73, pp. 147-51 and Fifth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5) 2001, E0032 (www.mdpi.net).
106. Kidwai, M.; Sapra, P. “An expeditious solventless synthesis of isoxazoles.” Org. Prep. Proced. Intl. 2001, 33, pp. 381-86.
107. Kidwai, M.; Sapra, P.; Misra, P.; Saxena, R.K.; Singh, M. “Microwave-assisted solid support synthesis of novel 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazepines as potent antimicrobial agents.” Bioorg. Med. Chem. 2001, 9, pp. 217-220.
108. Kidwai, M.; Sapra, P.; Bhushan, K.R.; Misra, P. “Microwave-assisted solid support synthesis of pyrazolino/iminopyrimidino/thioxopyrimidino imidazolines.” Synthesis 2001, 10, pp. 1509-12.
109. Kidwai, M.; Sapra, P.; Bhushan, K.R.; Misra, P. “Microwave-assisted synthesis of novel 1,2,4-triazines in ‘dry media’.” Synth. Commun. 2001, 31, pp. 1639-45.
110. Kidwai, M., Bhushan, K.R.; Sapra, P.; Saxena, R.K.; Gupta, R. “Alumina-supported synthesis of antibacterial quinolines using microwaves.” Bioorg. Med. Chem. 2000, 8, pp. 69-72.
111. Kidwai, M.; Venkataramanan, R.; Kohli, S. “Alumina-supported synthesis of β-lactams using microwave.” Synth. Commun. 2000, 30, pp. 989-1002.
112. Kidwai, M.; Misra, P.; Dave, B.; Bhushan, K.R.; Saxena, R.K.; Singh, M. “Microwave-activated solid support synthesis of new antibacterial quinolones.” Monatsh. Chem. 2000, 131, pp. 1207-12.
113. Kidwai, M.; Misra, P.; Bhushan, K.R. “Microwave assisted synthesis of novel organomercurials in ‘dry media’.” Polyhedron 1999, 18, pp. 2641-43.
114. Lange, J.H.M.; Verveer, P.C.; Osnabrug, S.J.M.; Visser, G.M. “Rapid microwave-enhanced synthesis of 4-hydroxy-quinolinones under solvent-free conditions.” Tetrahedron Lett. 2001, 42, pp. 1367-69.
115. Li, J.P.; Luo, Q.F.; Song, Y.M.; Wang, Y.L. “A rapid and efficient synthesis of diaryl thioureas via solvent-free reaction using microwave.” Chinese Chem. Lett. 2001, 12, pp. 383-86.
116. Li, J.P.; Luo, Q.F.; Wang, Y.L.; Wang, H. “Solvent-free synthesis of heterocyclic thioureas using microwave technology.” J. Chin. Chem. Soc. 2001, 48, pp. 73-75.
117. Wang, C.; Li, G.S.; Li, J.C.; Feng, S.; Li, X.L. “Synthesis of 5-alkylene barbituric acid in solventless system under microwave irradiation.” Chin. J. Org. Chem. 2001, 21, pp. 310-12.
118. Xu, Q.; Chao, B.; Wang, Y.; Dittmer, D.C. “Tellurium in the “no-solvent” organic synthesis of allylic alcohols.” Tetrahedron 1997, 53, pp. 12131-146.
119. Loghmani-Khouzani, H.; Sadeghi, M.M.; Safari, J.; Minaeifar, A. “A novel method for the synthesis of 2-ketomethylquinolines under solvent-free conditions using microwave irradiation.” Tetrahedron Lett. 2001, 42, pp. 4363-64.
120. Loghmani-Khouzani, H.; Sadeghi, M.M.; Safari, J.; Abdorrezaie, M.S.; Jafarpisheh, M. “A convenient synthesis of azines under solvent-free conditions using microwave irradiation.” J. Chem. Res. (S) 2001, pp. 80-81.
121. Shaabani, A.; Bahadoran, F.; Bazgir, A.; Safari, N. “Synthesis of metallophthalocyanines under solvent-free conditions using microwave irradiation.” Iranian J. Chem. & Chem. Eng. (Intl. Engl. Ed.) 1999, 18, pp. 104-07.
122. Maree, M.D.; Nyokong, T. “Solvent-free axial ligand substitution in octaphenoxyphthalocyaninato silicon complexes using microwave irradiation.” J. Chem. Res. (S) 2001, pp. 68-69.
123. Massicot, F.; Plantier-Royon, R.; Portella, C.; Saleur, D.; Sudha, A.V.R.L. “Solvent-free synthesis of tartramides under microwave activation.” Synthesis 2001, pp. 2441-44.
124. Mirjalili, B.F.; Zolfigol, M.A.; Bamoniri, A. “Silica sulfuric acid/wet SiO2 as a novel system for the deprotection of acetals by using microwave irradiation under solvent free conditions.” J. Korean Chem. Soc. 2001, 45, pp. 546-48.
125. Pezet, F.; Sasaki, I.; Daran, J.C.; Hydrio, J.; Ait-Haddou, H.; Balavoine, G. “First example of supported microwave-assisted synthesis of new chiral bipyridines and a terpyridine - use in asymmetric cyclopropanation.” Eur. J. Inorg. Chem. 2001, pp. 2669-74.
126. Quiroga, J.; Cisneros, C.; Insuasty, B.; Abonia, R.; Nogueras, M.; Sanchez, A. “A regiospecific three-component one-step cyclocondensation to 6-cyano-5,8-dihydropyrido-[2,3-d]pyrimidin-4(3H)-ones using microwaves under solvent-free conditions.” Tetrahedron Lett. 2001, 42, pp. 5625-27.
127. Rajakumar, P.; Murali, V. “Sodium sulfate supported synthesis of cationic cyclophanes using microwaves.” J. Chem. Soc., Chem. Commun. 2001, pp. 2710-11.
128. Romanova, N.N.; Kudan, P.V.; Gravis, A.G.; Zyk, N.V. “Investigation of the stereochemistry of rapid solvent-free microwave syntheses of β-amino acid esters.” Fifth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5) 2001, E0018 (www.mdpi.net).
129. Romanova, N.N.; Gravis, A.G.; Kudan, P.V.; Bundel, Y.G. “Solvent-free stereoselective synthesis of β-aryl-β-amino acid esters by the Rodionov reaction using microwave irradiation.” Mendeleev Commun. 2001, pp. 26-27.
130. Shanmugam, P.; Singh, P.R. “Montmorillonite K-10 clay-microwave assisted isomerisation of acetates of the Baylis-Hillman adducts: a facile method of stereoselective synthesis of (E)-trisubstituted alkenes.” Synlett. 2001, 8, pp. 1314-16.
131. Vanden Eynde, J.J.; Hecq, N.; Kappe, C.O.; Kataeva, O. “Microwave-mediated regioselective synthesis of novel pyrimido[1,2-a]pyrimidines under solvent-free conditions.” Tetrahedron 2001, 57, pp. 1785-91 and Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4) 2000, A0050 (www.mdpi.net).
132. a) Villemin, D.; Hammadi, M.; Hachemi, M.; Bar, N. “Applications of microwave in organic synthesis: an improved one-step synthesis of metallophthalocyanines and a new modified microwave oven for dry reaction.” Fifth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5) 2001, E0046 (www.mdpi.net). b) Villemin, D.; Hammadi, M.; Hachemi, M. Synth. Commun. 2002, 32, in press.
133. Villemin, D.; Hammadi, M.; Martin, B. “Clay catalysis: condensation of orthoesters with o-substituted aminoaromatics into heterocycles.” Synth. Commun. 1996, 26, pp. 2895-99.
134. Villemin, D.; Hammadi, M. “Environmentally desirable synthesis without the use of organic solvent. Synthesis of aryloxyacetic acids.” Synth. Commun. 1996, 26, pp. 4337-41.
135. Villemin, D.; Hachemi, M.; Lalaoui, M. “Potassium fluoride on alumina: synthesis of O-aryl-N,N-dimethylthiocarbamates and their rearrangement into S-aryl-N,N-dimethylthiocarbamates under microwave irradiation.” Synth. Commun. 1996, 26, pp. 2461-71.
136. Villemin, D.; Martin, B. “Dry condensation of creatinine with aldehydes under focused microwave irradiation.” Synth. Commun. 1995, 25, pp. 3135-40.
137. Villemin, D.; Hammadi, M. “Oxidation by DMSO. II. Opening of epoxides into a-hydroxyketones in presence of KSF clay under microwave irradiation.” Synth. Commun. 1995, 25, pp. 3141-44.
138. Yadav, J.S.; Reddy, B.V.S.; Madan, C. “Microwave-assisted efficient one-pot synthesis of nitriles in dry media.” J. Chem. Res. (S) 2001, pp. 190-91.
139. Yadav, J.S.; Reddy, B.V.S.; Madan, C. “Montmorillonite clay-catalyzed one-pot synthesis of α-amino phosphonates.” Synlett. 2001, 7, pp. 1131-33.
140. Yadav, J.S.; Reddy, B.V.S.; Madan, C. “Montmorillonite clay-catalyzed stereoselective syntheses of aryl-substituted (E)- and (Z)-allyl iodides and bromides.” New J. Chem. 2001, 25, pp. 1114-17.
141. Meshram, H.M.; Sekhar, K.C.; Ganesh, Y.S.S.; Yadav, J.S. “Clay catalyzed facile cyclodehydration under microwave: synthesis of 3-substituted benzofurans.” Synlett. 2000, 9, pp. 1273-74.
142. Ramalingam, T.; Reddy, B.V.S.; Srinivas, R.; Yadav, J.S. “Solvent-free conversion of N,N-dimethylhydrazones to nitriles under microwave irradiation.” Synth. Commun. 2000, 30, pp. 4507-12.
143. Kumar, H.M.S.; Reddy, B.V.S.; Anjaneyulu, S.; Reddy, E.J.; Yadav, J.S. “Clay catalysed amidation of alcohols with nitriles in dry media.” New J. Chem. 1999, pp. 955-56.
144. Sabitha, G.; Babu, R.S.; Yadav, J.S. “One pot synthesis of 4-(2-hydroxybenzoyl) pyrazoles from 3-formylchromones under microwave irradiation in solvent free conditions.” Synth. Commun. 1998, 28, pp. 4571-76.
145. Bandgar, B.P.; Kasture, S.P. “Microwave-induced solvent-free, rapid and efficient synthesis of conjugated nitroalkenes using sulfated zirconia.” Indian J. Chem., Sect. B 2001, 40, pp. 1239-41.
146. Bandgar, B.P.; Makone, S.S. “Rapid and selective regeneration of carbonyl compounds from their oximes under mild, neutral and solvent-free conditions.” Org. Prep. Proced. Intl. 2000, 32, pp. 391-94.
147. Bandgar, B.P.; Uppalla, L.S.; Kurule, D.S. “Solvent-free one-pot rapid synthesis of 3-carboxycoumarins - using focused microwaves.” Green Chem. 1999, 1, pp. 243-45.
148. Michaud, D.; Abdallah-El Ayoubi, S.; Dozias, M.J.; Toupet, L.; Texier-Boullet, F.; Hamelin, J. “New route to functionalized cyclohexenes from nitromethane and electrophilic alkenes without solvent under focused microwave irradiation.” J. Chem. Soc., Chem. Commun. 1997, pp. 1613-14.
149. Kasmi, S.; Hamelin, J.; Benhaoua, H. “Microwave-assisted solvent-free synthesis of iminothiazolines.” Tetrahedron Lett. 1998, 39, pp. 8093-96.
150. Perio, B.; Dozias, M.J.; Hamelin, J. “Ecofriendly fast batch synthesis of dioxolanes, dithiolanes, and oxathiolanes without solvent under microwave irradiation.” Org. Process Res. Dev. 1998, 2, pp. 428-30.
151. Saoudi, A.; Hamelin, J.; Benhaoua, H. “Elimination reaction over solid supports under microwave irradiation: synthesis of functionalized alkenes.” Tetrahedron Lett. 1998, 39, pp. 4035-38.
152. Meddad, N.; Rahmouni, M.; Derdour, A.; Bazureau, J.P.; Hamelin, J. “Eco-friendly transamination and aza-annulation reactions: solvent-free synthesis of new α-hetero-β-hydrazino acrylates and 1,2-dihydropyrazol-3-ones.” Synthesis 2001, pp. 581-84.
153. Benhaliliba, H.; Derdour, A.; Bazureau, J.P.; Texier-Boullet, F.; Hamelin, J. “Solvent free oxidation of β,β-disubstituted enamines under microwave irradiation.” Tetrahedron Lett. 1998, 39, pp. 541-42.
154. Dahmani, Z.; Rahmouni, M.; Brugidou, R.; Bazureau, J.P.; Hamelin, J. “A new route to α-hetero-β-enamino esters using a mild and convenient solvent-free process assisted by focused microwave irradiation.” Tetrahedron Lett. 1998, 39, pp. 8453-56.
155. Kerneur, G.; Lerestif, J.M.; Bazureau, J.P.; Hamelin, J. “Convenient preparation of 4-alkylidene-lH-imidazol-5(4H)-one derivatives from imidate and aldehydes by a solvent-free cycloaddition under microwaves.” Synthesis 1997, 3, pp. 287-89.
156. Cherouvrier, J.R.; Bazureau, J.P. “A practical and stereo-selective route to 5-ylidene-3,5-dihydroimidazol-4-one derivatives using solvent-free conditions under focused microwave irradiations.” Fifth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5) 2001, E0012 (www.mdpi.net).
157. Fraga-Dubreuil, J.; Cherouvrier, J.R.; Bazureau, J.P. “Clean solvent-free dipolar cycloaddition reactions assisted by focused microwave irradiations for the synthesis of new ethyl 4-cyano-2-oxazoline-4-carboxylates.” Green Chem. 2000, 2, pp. 226-29.
158. Gianotti, M.; Martelli, G.; Spunta, G.; Campana, E.; Panunzio, M.; Mendozza, M. “Solvent-free microwave-assisted organic reactions, preparation of β-keto-esters.” Synth. Commun. 2000, 30, pp. 1725-30.
159. Esteves-Souza, A.; Echevarria, A.; Vencato, I.; Jimeno, M.L.; Elguero, J. “Unexpected formation of bis-pyrazolyl derivatives by solid support coupled with microwave irradiation.” Tetrahedron 2001, 57, pp. 6147-53.
160. Gomez-Lara, J.; Gutierrez-Perez, R.; Penieres-Carrillo, G.; Lopez-Cortes, J.G.; Escudero-Salas, A.; Alvarez-Toledano, C. “Reaction of hydroquinones with supported oxidizing reagents in solvent-free conditions.” Synth. Commun. 2000, 30, pp. 2713-20.
161. Lopez-Cortes, J.G.; Penieres-Carrillo, G.; Ortega-Alfaro, M.C.; Gutierrez-Perez, R.; Toscano, R.A.; Alvarez-Toledano, C. “Oxidative coupling-type mechanism of N,N-dialkylanilines in solvent-free conditions forming crystal violet derivatives. A clay-mediated and microwave-promoted approach.” Can. J. Chem. 2000, 78, pp. 1299-304.
162. Rico-Gomez, R.; Najera, F.; Lopez-Romero, J.M.; Canada-Rudner, P. “Solvent-free synthesis of thio-alkylxanthines from alkylxanthines using microwave irradiation.” Heterocycles 2000, 53, pp. 2275-78.
163. Ley, S.V.; Baxendale, I.R.; Bream, R.N.; Jackson, P.S.; Leach, A.G.; Longbottom, D.A.; Nesi, M.; Scott, J.S.; Storer, R.I.; Taylor, S.J. “Multi-step organic synthesis using solid-supported reagents and scavengers: a new paradigm in chemical library generation.” J. Chem. Soc., Perkin Trans. 1 2000, pp. 3815-4195.
164. Habermann, J.; Ley, S.V.; Scott, J.S. “Synthesis of the potent analgesic compound (±)-epibatidine using an orchestrated multi-step sequence of polymer supported reagents.” J. Chem. Soc., Perkin Trans. 1 1999, pp. 1253-56.
165. Olsson, R.; Hansen, H.C.; Andersson, C.M. “Microwave-assisted solvent-free parallel synthesis of thioamides.” Tetrahedron Lett. 2000, 41, pp. 7947-50.
166. Sauvagnat, B.; Lamaty, F.; Lazaro, R.; Martinez, J. “Poly(ethyleneglycol) as solvent and polymer support in the microwave assisted parallel synthesis of amino acid derivatives.” Tetrahedron Lett. 2000, 41, pp. 6371-75.
167. Varray, S.; Sauvagnat, B.; Gauzy, C.; Lamaty, F.; Lazaro, R.; Martinez, J. “Poly(ethyleneglycol) supported synthesis of amino acid derivatives via ring closing metathesis or microwave-assisted alkylation.” Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4) 2000, B0010 (www.mdpi.net).
168. Sharifi, A.; Mohsenzadeh, F.; Naimi-Jamal, M.R. “Solvent-free preparation of monoacylaminals assisted by microwave irradiation.” J. Chem. Res. (S) 2000, pp. 394-96.
169. Stefani, H.A.; Gatti, P.M. “3,4-Dihydropyrimidin-2(1H)-ones: fast synthesis under microwave irradiation in solvent-free conditions.” Synth. Commun. 2000, 30, pp. 2165-73.
170. Tamami, B.; Kiasat, A.R. “Microwave promoted rapid oxidative deoximation of oximes under solvent-free conditions.” Synth. Commun. 2000, 30, pp. 4129-35.
171. Jnaneshwara, G.K.; Deshpande, V.H.; Bedekar, A.V. “Clay-catalyzed conversion of 2,2-disubstituted malononitriles to 2-oxazolines: towards unnatural amino acids.” J. Chem. Res. (S) 1999, pp. 252-53.
172. Mojtahedi, M.M.; Saidi, M.R.; Bolourtchian, M. “Microwave assisted aminolysis of epoxides under solvent-free conditions catalyzed by montmorillonite clay.” J. Chem. Res. (S) 1999, pp. 128-29.
173. Bogdal, D. “Coumarins - solvent free synthesis by the Knoevenagel condensation under microwave irradiation.” Electronic Conference on Trends in Heterocyclic Chemistry (ECTOC-4: ECHET98) 1998, Article 087 (www.ch.ic.ac.uk/ectoc/).
174. Bogdal, D.; Pielichowski, J.; Jaskot, K. “A rapid Williamson synthesis under microwave irradiation in dry media.” Org. Prep. Proc. Intl. 1998, 30, pp. 427-32.
175. Bogdal, D.; Pielichowski, J.; Boron, A. “Synthesis of aromatic ethers under microwave irradiation in dry media.” First International Electronic Conference on Synthetic Organic Chemistry (ECSOC-1) 1997, A0049 (www.mdpi.net).
176. Coville, N.J.; Cheng, L. “Organometallic chemistry in the solid state.” J. Organomet. Chem. 1998, 571, pp. 149-69.
177. Corsaro, A.; Chiacchio, U.; Librando, V.; Fisichella, S.; Pistara, V. “1,3-Dipolar cycloadditions of polycyclic aromatic hydrocarbons with nitrile oxides under microwave irradiation in the absence of solvent.” Heterocycles 1997, 45, pp. 1567-72.
178. Filip, S.V.; Nagy, G.; Surducan, E.; Surducan, V. “Microwave application in organic synthesis. Microwave-assisted preparation of diphenylamines in ‘dry media’.” First International Electronic Conference on Synthetic Organic Chemistry (ECSOC-1) 1997, A0031 (www.mdpi.net).
179. Nagy, G.; Filip, S.V.; Surducan, E.; Surducan, V. “Solvent-free synthesis of substituted phenoxyacetic acids under microwave irradiation.” Synth. Commun. 1997, 27, pp. 3729-36.
180. Kad, G.L.; Bhandari, M.; Kaur, J.; Rathee, R.; Singh, J. “Solventless preparation of oximes in the solid state and via microwave irradiation.” Green Chem. 2001, 3, pp. 275-77.
181. Kad, G.L.; Singh, V.; Kaur, K.P.; Singh, J. “Selective preparation of benzylic bromides in dry media coupled with microwave irradiation.” Tetrahedron Lett. 1997, 38, pp. 1079-80.
188. Larhed, M.; Hallberg, A. “Microwave-promoted palladium-catalyzed coupling reactions.” J. Org. Chem. 1996, 61, pp. 9582-84.
204. Combs, A.P.; Saubern, S.; Rafalski, M.; Lam, P.Y.S. “Solid supported aryl/heteroaryl C-N cross-coupling reactions.” Tetrahedron Lett. 1999, 40, pp. 1623-26.
276. Kidwai, M.; Venkataramanan, R.; Kohli, S. “Alumina-supported synthesis of β-lactams using microwave.” Synth. Commun. 2000, 30, pp. 989-1002.
297. Lipifiska, T.; Guibe-Jampel, E.; Petit, A.; Loupy, A. “2-(2-Pyridyl)indole derivatives preparation via Fischer reaction on montmorillonite K10/zinc chloride under microwave irradiation.” Synth. Commun. 1999, 29, pp. 1349-54.
290. Kidwai, M.; Misra, P. “Microwave-induced “solvent-free” novel technique for the synthesis of spiro[indole-pyrazole/isoxazole/pyrimidine] derivatives.” Oxidation Commun. 2001, 24, pp. 287-90.
310. Bougrin, K.; Loupy, A.; Petit, A.; Daou, B.; Soufiaoui, M. “Novel synthesis of 2-trifluoromethylarylimidazoles on montmorillonite K-10 in a ‘dry medium’ under microwave irradiation.” Tetrahedron 2001, 57, pp. 163-68.
351. Majdoub, M.; Loupy, A.; Petit, A.; Roudesli, S. “Coupling focused microwaves and solvent-free phase transfer catalysis: application to the synthesis of new furanic diethers.” Tetrahedron, 1996, 52, pp. 617-28.
364. Baruah, B.; Prajapati, D.; Boruah, A.; Sandhu, J.S. “Microwave induced 1,3-dipolar cycloaddition reactions of nitrones.” Synth. Commun. 1997, 27, pp. 2563-67.
416. Marquez, H.; Perez, E.R.; Plutin, A.M.; Morales, M.; Loupy, A. “Synthesis of 1-benzoyl-3-alkylthioureas by transamidation under microwave in dry media.” Tetrahedron Lett. 2000, 41, pp. 1753-56.
425. Oussaid, A.; Pentek, E.; Loupy, A. “Selective alkylations of 2-naphthol using solvent-free conditions under microwave irradiation.” New J. Chem. 1997, 21, pp. 1339-45.
439. Almena, I.; Diaz-Ortiz, A.; Diez-Barra, E.; de la Hoz, A.; Loupy, A. “Solvent-free benzylations of 2-pyridone. Regiospecific N- or C-alkylation.” Chem. Lett. 1996, pp. 333-34.
472. Chatti, S.; Bortolussi, M.; Loupy, A. “Synthesis of diethers derived from dianhydrohexitols by phase transfer catalysis under microwave.” Tetrahedron Lett. 2000, 41, pp. 3367-70.
491. Majdoub, M.; Loupy, A.; Petit, A.; Roudesli, S. “Coupling focused microwaves and solvent-free phase transfer catalysis: application to the synthesis of new furanic diethers.” Tetrahedron 1996, 52, pp. 617-28.
501. Gelo-Pujic, M.; Guibe-Jampel, E.; Loupy, A.; Galema, S.A.; Mathe, D. “Lipase-catalyzed esterification of some α-D-glucopyranosides in dry media using focused microwave irradiation.” J. Chem. Soc., Perkin Trans. 1 1996, pp. 2777-80.
505. Bram, G.; Loupy, A.; Majdoub, M. “Microwave irradiation plus solid-liquid phase transfer catalysis without solvent: further improvement in anionic activation.” Synth. Commun. 1990, 20, pp. 125-29.
506. Bram, G.; Loupy, A.; Majdoub, M.; Gutierrez, E.; Ruiz-Hitzky, E. “Alkylation of potassium acetate in ‘dry media’. Thermal activation in commercial microwave ovens.” Tetrahedron 1990, 46, pp. 5167-76.
507. Loupy, A.; Petit, A.; Ramdani, M.; Yvanaeff, C.; Majdoub, M.; Labiad, B.; Villemin, D. “The synthesis of esters under microwave irradiation using dry-media conditions.” Can. J. Chem. 1993, 71, pp. 90-95.
522. Bram, G.; Loupy, A.; Majdoub, M.; Petit, A. “Anthraquinone microwave-induced synthesis in dry media in domestic ovens.” Chem. Ind. (London) 1991, pp. 396-97.
568. Oussaid, A.; Loupy, A. “Selective oxidation of arenes in dry media under focused microwaves.” J. Chem. Res. (S) 1997, pp. 342-43.
590. Loupy, A.; Song, S.J.; Sohn, S.M.; Lee, Y.M.; Kwon, T.W. “Solvent-free bentonite-catalyzed condensation of malonic acid and aromatic aldehydes under microwave irradiation.” J. Chem. Soc., Perkin Trans. 1 2001, pp. 1220-22.
593. Abenhaim, D.; Son, C.P.N.; Loupy, A.; Nguyen, B.H. “Synthesis of jasminaldehyde by solid-liquid phase transfer catalysis without solvent, under microwave irradiation.” Synth. Commun. 1994, 24, pp. 1199-205.
607. de la Cruz, P.; Diez-Barra, E.; Loupy, A.; Langa, F. “Silica gel catalyzed Knoevenagel condensation in dry media under microwave irradiation.” Tetrahedron Lett. 1996, 37, pp. 1113-16.
609. Kuster, G.J.; Scheeren, H.W. “The preparation of resin-bound nitroalkenes and some applications in high pressure promoted cycloadditions.” Tetrahedron Lett. 2000, 41, pp. 515-19.
628. Kabalka, G.W.; Wang, L.; Pagni, R.M. “A microwave-enhanced, solventless Mannich condensation on CuI-doped alumina.” Synlett. 2001, pp. 676-78.
629. Kabalka, G.W.; Wang, L.; Pagni, R.M. “A novel route to 2-(dialkylaminomethyl) benzo[b]furans via a microwave-enhanced, solventless Mannich condensation-cyclization on cuprous iodide doped alumina.” Tetrahedron Lett. 2001, 42, pp. 6049-51.
631. Hoel, A.M.L.; Nielsen, J. “Microwave-assisted solid-phase Ugi four-component condensations.” Tetrahedron Lett. 1999, 40, pp. 3941-44.
657. Csiba, M.; Cleophax, J.; Loupy, A.; Malthete, J.; Gero, S.D. “Liquid crystalline 5,6-O-acetals of L-galactono-1,4-lactone prepared by microwave irradiation on montmorillonite.” Tetrahedron Lett. 1993, 34, pp. 1787-90.
708. Loupy, A.; Thach, L.N. “Base-catalyzed isomerization of eugenol: solvent-free conditions and microwave activation.” Synth. Commun. 1993, 23, pp. 2571-77.
709. Loupy, A.; Pigeon, P.; Ramdani, M.; Jacquault, P. “A new solvent-free procedure using microwave technology as an alternative to the Krapcho reaction.” J. Chem. Res. (S) 1993, pp. 36-37.

